The biology and mechanism of action of suppressor of cytokine signaling 3
- PMID: 22574771
- PMCID: PMC3757090
- DOI: 10.3109/08977194.2012.687375
The biology and mechanism of action of suppressor of cytokine signaling 3
Abstract
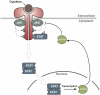
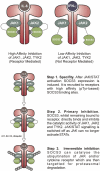
Suppressors of cytokine signaling 3 (SOCS3) has been shown to be an important and non-redundant feedback inhibitor of several cytokines including leukemia inhibitory factor, IL-6, IL-11, Ciliary neurotrophic factor (CNTF), leptin, and granulocyte colony-stimulating factor (G-CSF). Loss of SOCS3 in vivo has profound effects on placental development, inflammation, fat-induced weight gain, and insulin sensitivity. SOCS3 expression is induced by Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signaling and it then binds to specific cytokine receptors (including gp130, G-CSF, and leptin receptors). SOCS3 then inhibits JAK/STAT signaling in two distinct ways. First, SOCS3 is able to directly inhibit the catalytic activity of JAK1, JAK2, or TYK2 while remaining bound to the cytokine receptor. Second, SOCS3 recruits elongins B/C and Cullin5 to generate an E3 ligase that ubiquitinates both JAK and cytokine receptor targeting them for proteasomal degradation. Detailed in vivo studies have revealed that SOCS3 action not only limits the duration of cytokine signaling to prevent overactivity but it is also important in maintaining the specificity of cytokine signaling.
Figures


References
-
- Anderson P, Sundstedt A, Li L, O'Neill EJ, Li SL, Wraith DC, Wang P. Differential activation of signal transducer and activator of transcription (STAT)3 and STAT5 and induction of suppressors of cytokine signalling in T(h)1 and T(h)2 cells. International Immunology. 2003;15:1309–1317. - PubMed
-
- Babon JJ, McManus EJ, Yao SG, DeSouza DP, Mielke LA, Sprigg NS, Willson TA, Hilton DJ, Nicola NA, Baca M, Nicholson SE, Norton RS. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Molecular Cell. 2006;22:205–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
