Life- and person-centred help in Mecklenburg-Western Pomerania, Germany (DelpHi): study protocol for a randomised controlled trial
- PMID: 22575023
- PMCID: PMC3482148
- DOI: 10.1186/1745-6215-13-56
Life- and person-centred help in Mecklenburg-Western Pomerania, Germany (DelpHi): study protocol for a randomised controlled trial
Abstract
Background: The provision of appropriate medical and nursing care for people with dementia is a major challenge for the healthcare system in Germany. New models of healthcare provision need to be developed, tested and implemented on the population level. Trials in which collaborative care for dementia in the primary care setting were studied have demonstrated its effectiveness. These studies have been conducted in different healthcare systems, however, so it is unclear whether these results extend to the specific context of the German healthcare system.The objective of this population-based intervention trial in the primary care setting is to test the efficacy and efficiency of implementing a subsidiary support system on a population level for persons with dementia who live at home.
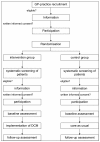
Methods and study design: The study was designed to assemble a general physician-based epidemiological cohort of people above the age of 70 who live at home (DelpHi cohort). These people are screened for eligibility to participate in a trial of dementia care management (DelpHi trial). The trial is a cluster-randomised, controlled intervention trial with two arms (intervention and control) designed to test the efficacy and efficiency of implementing a subsidiary support system for persons with dementia who live at home. This subsidiary support system is initiated and coordinated by a dementia care manager: a nurse with dementia-specific qualifications who delivers the intervention according to a systematic, detailed protocol. The primary outcome is quality of life and healthcare for patients with dementia and their caregivers. This is a multidimensional outcome with a focus on four dimensions: (1) quality of life, (2) caregiver burden, (3) behavioural and psychological symptoms of dementia and (4) pharmacotherapy with an antidementia drug and prevention or suspension of potentially inappropriate medication. Secondary outcomes include the assessment of dementia syndromes, activities of daily living, social support health status, utilisation of health care resources and medication.
Discussion: The results will provide evidence for specific needs in ambulatory care for persons with dementia and will show effective ways to meet those needs. Qualification requirements will be evaluated, and the results will help to modify existing guidelines and treatment paths.
Trial registration: NCT01401582.
Figures
References
-
- Fendrich K, Hoffmann W. More than just aging societies: the demographic change has an impact on actual numbers of patients. J Public Health. 2007;15:345–351. doi: 10.1007/s10389-007-0142-0. - DOI
-
- Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C, Frölich L, Riepe MW, Dodel R, Leyhe T, Bertram L, Hoffmann W. Faltraco F; German Task Force on Alzheimer’s Disease (GTF-AD): The future of Alzheimer’s disease: the next 10 years. Prog Neurobiol. 2011;95:718–728. doi: 10.1016/j.pneurobio.2011.11.008. - DOI - PubMed
-
- Bickel H. Deutsche Alzheimer Gesellschaft e.V.: Die Epidemiologie der Demenz. Vol. 1. Das Wichtigste-Informationsblatt der Deutschen Alzheimer Gesellschaft e.V, Berlin; 2008. [ http://www.deutsche-alzheimer.de/fileadmin/alz/pdf/factsheets/FactSheet0...]
-
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) Cholinesterasehemmer bei Alzheimer Demenz (Abschlussbericht A05-19A:) IQWiG, Köln; 2007. [ https://www.iqwig.de/download/A05-19A_Abschlussbericht_Cholinesterasehem...]
-
- Thyrian JR, Dreier A, Fendrich K, Lueke S, Hoffmann W. Demenzerkrankungen: Wirksame Konzepte gesucht. Dtsch Arztebl. 2011;108:A1954–A1956.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical