Cell size control in yeast
- PMID: 22575477
- PMCID: PMC3350643
- DOI: 10.1016/j.cub.2012.02.041
Cell size control in yeast
Abstract
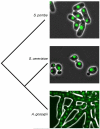
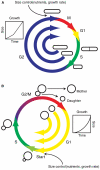
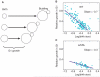
Cell size is an important adaptive trait that influences nearly all aspects of cellular physiology. Despite extensive characterization of the cell-cycle regulatory network, the molecular mechanisms coupling cell growth to division, and thereby controlling cell size, have remained elusive. Recent work in yeast has reinvigorated the size control field and suggested provocative mechanisms for the distinct functions of setting and sensing cell size. Further examination of size-sensing models based on spatial gradients and molecular titration, coupled with elucidation of the pathways responsible for nutrient-modulated target size, may reveal the fundamental principles of eukaryotic cell size control.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
-
- Thompson DAW, Whyte LL. On growth and form. Cambridge University Press; Cambridge: 1942.
-
- Payne JL, Groves JR, Jost AB, Nguyen T, Myhre S, Hill TM, Skotheim JS. Late Paleozoic fusulinoidean gigantism driven by atmospheric hyperoxia. - PubMed
-
- Payne JL, Jost AB, Wang SC, Skotheim JS. A permanent shift in the evolutionary dynamics of organism size caused by the end-Permian mass extinction.
-
- Gregory TR. Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol Rev CambPhilosSoc. 2001;76:65–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

