Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress
- PMID: 22575600
- PMCID: PMC3392533
- DOI: 10.1016/j.expneurol.2012.04.018
Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress
Abstract
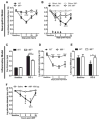
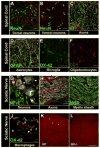
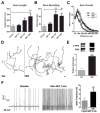
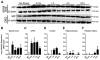
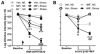
Stress and glucocorticoids exacerbate pain via undefined mechanisms. Macrophage migration inhibitory factor (MIF) is a constitutively expressed protein that is secreted to maintain immune function when glucocorticoids are elevated by trauma or stress. Here we show that MIF is essential for the development of neuropathic and inflammatory pain, and for stress-induced enhancement of neuropathic pain. Mif null mutant mice fail to develop pain-like behaviors in response to inflammatory stimuli or nerve injury. Pharmacological inhibition of MIF attenuates pain-like behaviors caused by nerve injury and prevents sensitization of these behaviors by stress. Conversely, injection of recombinant MIF into naïve mice produces dose-dependent mechanical sensitivity that is exacerbated by stress. MIF elicits pro-inflammatory signaling in microglia and activates sensory neurons, mechanisms that underlie pain. These data implicate MIF as a key regulator of pain and provide a mechanism whereby stressors exacerbate pain. MIF inhibitors warrant clinical investigation for the treatment of chronic pain.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


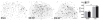
References
-
- Aloisi AM, Pari G, Ceccarelli I, Vecchi I, Ietta F, Lodi L, Paulesu L. Gender-related effects of chronic non-malignant pain and opioid therapy on plasma levels of macrophage migration inhibitory factor (MIF) Pain. 2005;115:142–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS047175/NS/NINDS NIH HHS/United States
- AI 064320/AI/NIAID NIH HHS/United States
- R01 NS059776/NS/NINDS NIH HHS/United States
- R56 AI090803/AI/NIAID NIH HHS/United States
- P30 NS045758/NS/NINDS NIH HHS/United States
- R21 AI076309/AI/NIAID NIH HHS/United States
- R01 NS037846/NS/NINDS NIH HHS/United States
- AI 076309/AI/NIAID NIH HHS/United States
- AI 090803/AI/NIAID NIH HHS/United States
- R56 AI068829/AI/NIAID NIH HHS/United States
- R01 GMK081658/PHS HHS/United States
- R01 AI064320/AI/NIAID NIH HHS/United States
- AI 068829/AI/NIAID NIH HHS/United States
- P30-NS045758/NS/NINDS NIH HHS/United States
- NS37846/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

