Site-specific chemical protein conjugation using genetically encoded aldehyde tags
- PMID: 22576105
- PMCID: PMC3498491
- DOI: 10.1038/nprot.2012.045
Site-specific chemical protein conjugation using genetically encoded aldehyde tags
Abstract
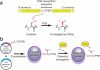
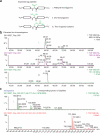
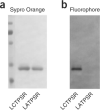
We describe a method for modifying proteins site-specifically using a chemoenzymatic bioconjugation approach. Formylglycine generating enzyme (FGE) recognizes a pentapeptide consensus sequence, CxPxR, and it specifically oxidizes the cysteine in this sequence to an unusual aldehyde-bearing formylglyine. The FGE recognition sequence, or aldehyde tag, can be inserted into heterologous recombinant proteins produced in either prokaryotic or eukaryotic expression systems. The conversion of cysteine to formylglycine is accomplished by co-overexpression of FGE, either transiently or as a stable cell line, and the resulting aldehyde can be selectively reacted with α-nucleophiles to generate a site-selectively modified bioconjugate. This protocol outlines both the generation and the analysis of proteins aldehyde-tagged at their termini and the methods for chemical conjugation to the formylglycine. The process of generating aldehyde-tagged protein followed by chemical conjugation and purification takes 20 d.
Figures
Similar articles
-
Site-Specific Bioconjugation Using SMARTag® Technology: A Practical and Effective Chemoenzymatic Approach to Generate Antibody-Drug Conjugates.Methods Mol Biol. 2019;2033:131-147. doi: 10.1007/978-1-4939-9654-4_10. Methods Mol Biol. 2019. PMID: 31332752
-
Eukaryotic formylglycine-generating enzyme catalyses a monooxygenase type of reaction.FEBS J. 2015 Sep;282(17):3262-74. doi: 10.1111/febs.13347. Epub 2015 Jul 7. FEBS J. 2015. PMID: 26077311
-
Leveraging Formylglycine-Generating Enzyme for Production of Site-Specifically Modified Bioconjugates.Methods Mol Biol. 2018;1728:3-16. doi: 10.1007/978-1-4939-7574-7_1. Methods Mol Biol. 2018. PMID: 29404988
-
Formylglycine-generating enzymes for site-specific bioconjugation.Biol Chem. 2019 Feb 25;400(3):289-297. doi: 10.1515/hsz-2018-0358. Biol Chem. 2019. PMID: 30291781 Review.
-
Formylglycine, a post-translationally generated residue with unique catalytic capabilities and biotechnology applications.ACS Chem Biol. 2015 Jan 16;10(1):72-84. doi: 10.1021/cb500897w. ACS Chem Biol. 2015. PMID: 25514000 Free PMC article. Review.
Cited by
-
Computational Construction of Antibody-Drug Conjugates Using Surface Lysines as the Antibody Conjugation Site and a Non-cleavable Linker.Cancer Inform. 2014 Dec 8;13:179-86. doi: 10.4137/CIN.S19222. eCollection 2014. Cancer Inform. 2014. PMID: 25506200 Free PMC article.
-
Methods to Make Homogenous Antibody Drug Conjugates.Pharm Res. 2015 Nov;32(11):3480-93. doi: 10.1007/s11095-014-1596-8. Epub 2014 Dec 16. Pharm Res. 2015. PMID: 25511917 Free PMC article. Review.
-
Targeting cancer with antibody-drug conjugates: Promises and challenges.MAbs. 2021 Jan-Dec;13(1):1951427. doi: 10.1080/19420862.2021.1951427. MAbs. 2021. PMID: 34291723 Free PMC article.
-
Site-Specific Antibody Conjugation for ADC and Beyond.Biomedicines. 2017 Nov 9;5(4):64. doi: 10.3390/biomedicines5040064. Biomedicines. 2017. PMID: 29120405 Free PMC article. Review.
-
LASIC: Light Activated Site-Specific Conjugation of Native IgGs.Bioconjug Chem. 2015 Aug 19;26(8):1456-60. doi: 10.1021/acs.bioconjchem.5b00275. Epub 2015 Jun 12. Bioconjug Chem. 2015. PMID: 26057140 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

