Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes
- PMID: 22576345
- PMCID: PMC7412749
- DOI: 10.1097/CJI.0b013e31825898c5
Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes
Abstract
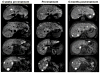
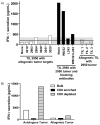
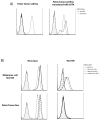
Adoptive cell therapy using tumor-infiltrating lymphocytes (TIL) can mediate objective and durable tumor regressions in patients with metastatic melanoma. CD8+ tumor-reactive TIL are well studied in humans and animals, yet the function of tumor-infiltrating CD4+ T lymphocytes in patient treatments remains controversial. We recently demonstrated that CD4+ TILs are not necessary for objective responses in patients. Coinfusion with tumor-specific CD4 TIL may enhance or increase the durability of tumor regressions, but the number of patients with tumor-reactive CD4 TIL is unknown. We screened 44 CD8+-depleted TIL for in vitro reactivity against autologous tumor. Nine (20%) showed specific reactivity by interferon-γ release assay, of which 8 were specifically blocked by an anti-HLA-DR antibody. Flow-cytometric analysis of these reactive TIL confirmed a high CD4+ composition (median 89%). Highlighting the contribution of CD4+ TIL to tumor regression, a patient with widespread metastatic disease was administered TIL containing HLA class II-restricted tumor activity with high-dose interleukin-2 therapy after lymphodepletion that mediated regression of extensive metastatic disease in the liver and spleen. These results demonstrate that at least 20% of metastatic melanomas contain CD4+ lymphocytes with specific tumor recognition and suggest a possible role for CD4+ cells in the effectiveness of adoptive cell therapy.
Figures

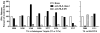
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

