The BLM helicase contributes to telomere maintenance through processing of late-replicating intermediate structures
- PMID: 22576367
- PMCID: PMC3424559
- DOI: 10.1093/nar/gks407
The BLM helicase contributes to telomere maintenance through processing of late-replicating intermediate structures
Abstract
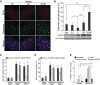
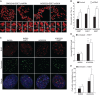
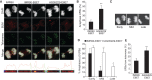
Werner's syndrome (WS) and Bloom's syndrome (BS) are cancer predisposition disorders caused by loss of function of the RecQ helicases WRN or BLM, respectively. BS and WS are characterized by replication defects, hyperrecombination events and chromosomal aberrations, which are hallmarks of cancer. Inefficient replication of the G-rich telomeric strand contributes to chromosome aberrations in WS cells, demonstrating a link between WRN, telomeres and genomic stability. Herein, we provide evidence that BLM also contributes to chromosome-end maintenance. Telomere defects (TDs) are observed in BLM-deficient cells at an elevated frequency, which is similar to cells lacking a functional WRN helicase. Loss of both helicases exacerbates TDs and chromosome aberrations, indicating that BLM and WRN function independently in telomere maintenance. BLM localization, particularly its recruitment to telomeres, changes in response to replication dysfunction, such as in WRN-deficient cells or after aphidicolin treatment. Exposure to replication challenge causes an increase in decatenated deoxyribonucleic acid (DNA) structures and late-replicating intermediates (LRIs), which are visible as BLM-covered ultra-fine bridges (UFBs) in anaphase. A subset of UFBs originates from telomeric DNA and their frequency correlates with telomere replication defects. We propose that the BLM complex contributes to telomere maintenance through its activity in resolving LRIs.
Figures

References
-
- de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. - PubMed
-
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. - PubMed
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. - PubMed
-
- Opresko PL, Cheng WH, von Kobbe C, Harrigan JA, Bohr VA. Werner syndrome and the function of the Werner protein; what they can teach us about the molecular aging process. Carcinogenesis. 2003;24:791–802. - PubMed

