Immunotherapy of cancer in 2012
- PMID: 22576456
- PMCID: PMC3445708
- DOI: 10.3322/caac.20132
Immunotherapy of cancer in 2012
Abstract
The immunotherapy of cancer has made significant strides in the past few years due to improved understanding of the underlying principles of tumor biology and immunology. These principles have been critical in the development of immunotherapy in the laboratory and in the implementation of immunotherapy in the clinic. This improved understanding of immunotherapy, enhanced by increased insights into the mechanism of tumor immune response and its evasion by tumors, now permits manipulation of this interaction and elucidates the therapeutic role of immunity in cancer. Also important, this improved understanding of immunotherapy and the mechanisms underlying immunity in cancer has fueled an expanding array of new therapeutic agents for a variety of cancers. Pegylated interferon-α2b as an adjuvant therapy and ipilimumab as therapy for advanced disease, both of which were approved by the United States Food and Drug Administration for melanoma in March 2011, are 2 prime examples of how an increased understanding of the principles of tumor biology and immunology have been translated successfully from the laboratory to the clinical setting. Principles that guide the development and application of immunotherapy include antibodies, cytokines, vaccines, and cellular therapies. The identification and further elucidation of the role of immunotherapy in different tumor types, and the development of strategies for combining immunotherapy with cytotoxic and molecularly targeted agents for future multimodal therapy for cancer will enable even greater progress and ultimately lead to improved outcomes for patients receiving cancer immunotherapy.
Copyright © 2012 American Cancer Society, Inc.
Figures




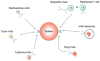



References
-
- Kim CJ, Dessureault S, Gabrilovich D, Reintgen DS, Slingluff CL., Jr Immunotherapy for melanoma. Cancer Control. 2002;9:22–30. - PubMed
-
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(suppl 1):S11–S14. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. - PubMed
-
- Dutcher JP, Creekmore S, Weiss GR, et al. A phase II study of interleukin-2 and lymphokine-activated killer cells in patients with metastatic malignant melanoma. J Clin Oncol. 1989;7:477–485. - PubMed
-
- Parkinson DR, Abrams JS, Wiernik PH, et al. Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J Clin Oncol. 1990;8:1650–1656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA112198/CA/NCI NIH HHS/United States
- R01 CA105500/CA/NCI NIH HHS/United States
- P01 CA101944/CA/NCI NIH HHS/United States
- CA114931/CA/NCI NIH HHS/United States
- CA110249/CA/NCI NIH HHS/United States
- P01 CA109688/CA/NCI NIH HHS/United States
- CA138635/CA/NCI NIH HHS/United States
- CA095128/CA/NCI NIH HHS/United States
- R01 CA112198/CA/NCI NIH HHS/United States
- R01 CA090360/CA/NCI NIH HHS/United States
- CA157467/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- R01 CA095128/CA/NCI NIH HHS/United States
- R01 CA138188/CA/NCI NIH HHS/United States
- CA109688/CA/NCI NIH HHS/United States
- P50 CA121973/CA/NCI NIH HHS/United States
- CA47904/CA/NCI NIH HHS/United States
- R01 CA110249/CA/NCI NIH HHS/United States
- R01 CA138635/CA/NCI NIH HHS/United States
- U10 CA039229/CA/NCI NIH HHS/United States
- CA39229/CA/NCI NIH HHS/United States
- CA132714/CA/NCI NIH HHS/United States
- R01 CA104947/CA/NCI NIH HHS/United States
- P30CA47904/CA/NCI NIH HHS/United States
- CA138188/CA/NCI NIH HHS/United States
- R01 CA157467/CA/NCI NIH HHS/United States
- P01 CA132714/CA/NCI NIH HHS/United States
- CA105500/CA/NCI NIH HHS/United States
- CA90360/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- R21 CA114931/CA/NCI NIH HHS/United States
- CA121973/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

