In vivo X-ray digital subtraction and CT angiography of the murine cerebrovasculature using an intra-arterial route of contrast injection
- PMID: 22576899
- PMCID: PMC7964744
- DOI: 10.3174/ajnr.A3071
In vivo X-ray digital subtraction and CT angiography of the murine cerebrovasculature using an intra-arterial route of contrast injection
Abstract
Background and purpose: Investigation of the anatomy, patency, and blood flow of arterial and venous vessels in small animal models of cerebral ischemia, venous thrombosis, or vasospasm is of major interest. However, due to their small caliber, in vivo examination of these vessels is technically challenging. Using micro-CT, we compared the feasibility of in vivo DSA and CTA of the murine cerebrovasculature using an intra-arterial route of contrast administration.
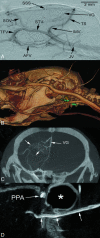
Materials and methods: The ECA was catheterized in 5 C57BL/6J mice. During intra-arterial injection of an iodized contrast agent (30 μL/1 sec), DSA of the intra- and extracranial vessels was performed in mice breathing room air and repeated in hypoxic/hypercapnic mice. Micro-CTA was performed within 20 seconds of intra-arterial contrast injection (220 μL/20 sec). Image quality of both methods was compared. Radiation dose measurements were performed with thermoluminescence dosimeters.
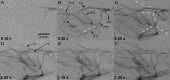
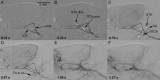
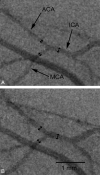
Results: Both methods provided high-resolution images of the murine cerebrovasculature, with the smallest identifiable vessel calibers of ≤ 50 μm. Due to its high temporal resolution of 30 fps, DSA allowed identification of anastomoses between the ICA and ECA by detection of retrograde flow within the superficial temporal artery. Micro-CTA during intra-arterial contrast injection resulted in a reduced injection volume and a higher contrast-to-noise ratio (19.0 ± 1.0) compared with DSA (10.0 ± 1.8) or micro-CTA when using an intravenous injection route (1.3 ± 0.4).
Conclusions: DSA of the murine cerebrovasculature is feasible using micro-CT and allows precise and repeated measurements of the vessel caliber, and changes of the vessel caliber, while providing relevant information on blood flow in vivo.
Figures



References
-
- Barone FC, Knudsen DJ, Nelson AH, et al. . Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab 1993; 13: 683– 92 - PubMed
-
- Zhang Z, Chopp M, Zhang RL, et al. . A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab 1997; 17: 1081– 88 - PubMed
-
- Kim DE, Schellingerhout D, Jaffer FA, et al. . Near-infrared fluorescent imaging of cerebral thrombi and blood-brain barrier disruption in a mouse model of cerebral venous sinus thrombosis. J Cereb Blood Flow Metab 2005; 25: 226– 33 - PubMed
-
- Sabri M, Jeon H, Ai J, et al. . Anterior circulation mouse model of subarachnoid hemorrhage. Brain Res 2009; 1295: 179– 85 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
