A combined early cognitive and physical rehabilitation program for people who are critically ill: the activity and cognitive therapy in the intensive care unit (ACT-ICU) trial
- PMID: 22577067
- PMCID: PMC3513484
- DOI: 10.2522/ptj.20110414
A combined early cognitive and physical rehabilitation program for people who are critically ill: the activity and cognitive therapy in the intensive care unit (ACT-ICU) trial
Abstract
Background: In the coming years, the number of survivors of critical illness is expected to increase. These survivors frequently develop newly acquired physical and cognitive impairments. Long-term cognitive impairment is common following critical illness and has dramatic effects on patients' abilities to function autonomously. Neuromuscular weakness affects similar proportions of patients and leads to equally profound life alterations. As knowledge of these short-term and long-term consequences of critical illness has come to light, interventions to prevent and rehabilitate these devastating consequences have been sought. Physical rehabilitation has been shown to improve functional outcomes in people who are critically ill, but subsequent studies of physical rehabilitation after hospital discharge have not. Post-hospital discharge cognitive rehabilitation is feasible in survivors of critical illness and is commonly used in people with other forms of acquired brain injury. The feasibility of early cognitive therapy in people who are critically ill remains unknown.
Objective: The purpose of this novel protocol trial will be to determine the feasibility of early and sustained cognitive rehabilitation paired with physical rehabilitation in patients who are critically ill from medical and surgical intensive care units.
Design: This is a randomized controlled trial.
Setting: The setting for this trial will be medical and surgical intensive care units of a large tertiary care referral center.
Patients: The participants will be patients who are critically ill with respiratory failure or shock.
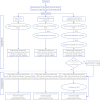
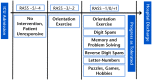
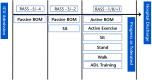
Intervention: Patients will be randomized to groups receiving usual care, physical rehabilitation, or cognitive rehabilitation plus physical rehabilitation. Twice-daily cognitive rehabilitation sessions will be performed with patients who are noncomatose and will consist of orientation, memory, and attention exercises (eg, forward and reverse digit spans, matrix puzzles, letter-number sequences, pattern recognition). Daily physical rehabilitation sessions will advance patients from passive range of motion exercises through ambulation. Patients with cognitive or physical impairment at discharge will undergo a 12-week, in-home cognitive rehabilitation program.
Measurements: A battery of neurocognitive and functional outcomes will be measured 3 and 12 months after hospital discharge.
Conclusions: If feasible, these interventions will lay the groundwork for a larger, multicenter trial to determine their efficacy.
Trial registration: ClinicalTrials.gov NCT01270269.
Figures
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310 - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693 - PubMed
-
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377 - PubMed
-
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- P30AG21342/AG/NIA NIH HHS/United States
- P30 AG021342/AG/NIA NIH HHS/United States
- R01 AG035117/AG/NIA NIH HHS/United States
- K24AG021507/AG/NIA NIH HHS/United States
- AG027472/AG/NIA NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- AG034257/AG/NIA NIH HHS/United States
- 1 UL1 RR024975/RR/NCRR NIH HHS/United States
- T32HL087738/HL/NHLBI NIH HHS/United States
- K23 AG031322/AG/NIA NIH HHS/United States
- K23 AG034257/AG/NIA NIH HHS/United States
- AG031322/AG/NIA NIH HHS/United States
- AG035117/AG/NIA NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- R01 AG027472/AG/NIA NIH HHS/United States
- K24 AG021507/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

