ZNF764 haploinsufficiency may explain partial glucocorticoid, androgen, and thyroid hormone resistance associated with 16p11.2 microdeletion
- PMID: 22577170
- PMCID: PMC3410270
- DOI: 10.1210/jc.2011-3493
ZNF764 haploinsufficiency may explain partial glucocorticoid, androgen, and thyroid hormone resistance associated with 16p11.2 microdeletion
Abstract
Context: Nuclear hormone receptors exert their transcriptional effects through shared cofactor molecules; thus, defects in such intermediate proteins may be associated with multiple hormone resistance. Microdeletion of small chromosomal segments results in hereditary or sporadic diseases by affecting expression of residing genes.
Objectives: We describe a 7-yr-old boy with partial resistance to glucocorticoids, thyroid hormones, and possibly androgens. He was diagnosed as being in the autism spectrum disorder and had developmental delay and several facial morphological manifestations. We explored genes responsible for multiple hormone resistance of this case.
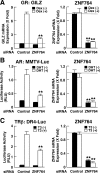
Results: We found in this patient an approximately 1.1-Mb heterozygous 16p11.2 microdeletion, which included an approximately 500-kb unique deletion along with the common, previously reported approximately 600-kb 16p11.2 microdeletion. The small interfering RNA-based screening revealed that knockdown of ZNF764, which is located in the deleted segment unique to our case, significantly reduced glucocorticoid-, androgen-, and thyroid hormone-induced transcriptional activity of their responsive genes in HeLa cells, whereas its overexpression enhanced their transcriptional activity. The activities of the estrogen and progesterone receptors, cAMP response element-binding protein, and p53 were not affected in these cells. ZNF764 (zinc finger protein 764) expression was reduced in the patient's peripheral blood mononuclear cells, whereas exogenously supplemented ZNF764 recovered responsiveness to glucocorticoids in the patient's Epstein-Barr virus-transformed lymphocytes. The effect of ZNF764 on the glucocorticoid receptor transcriptional activity was mediated through cooperation with a general nuclear hormone receptor coactivator, transcriptional intermediary factor 1.
Conclusions: ZNF764 haploinsufficiency caused by microdeletion may be responsible for the partial multiple hormone resistance observed in our patient. ZNF764 appears to be involved in glucocorticoid, androgen, and thyroid hormone action.
Figures




References
-
- Larsen PR, Ingbar SH. 1998. The thyroid gland. In: Wilson JD, Foster DW, eds. Williams textbook of endocrinology. Philadelphia: WB Saunders; 357–487
-
- Kovacs WJ, Orth DN. 1998. The adrenal cortex. In: Wilson JD, Foster DW, eds. Williams textbook of endocrinology. Philadelphia: WB Saunders; 517–750
-
- Griffin JE, Wilson JD. 1998. Disorders of the testis and the male reproductive tract. In: Wilson JD, Foster DW. eds. Williams textbook of endocrinology. Philadelphia: WB Saunders; 799–852
-
- McKenna NJ, Lanz RB, O'Malley BW. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 - PubMed
-
- New MI, Nimkarn S, Brandon DD, Cunningham-Rundles S, Wilson RC, Newfield RS, Vandermeulen J, Barron N, Russo C, Loriaux DL, O'Malley B. 1999. Resistance to several steroids in two sisters. J Clin Endocrinol Metab 84:4454–4464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

