Examining form and function of dendritic spines
- PMID: 22577585
- PMCID: PMC3345238
- DOI: 10.1155/2012/704103
Examining form and function of dendritic spines
Abstract
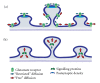
The majority of fast excitatory synaptic transmission in the central nervous system takes place at protrusions along dendrites called spines. Dendritic spines are highly heterogeneous, both morphologically and functionally. Not surprisingly, there has been much speculation and debate on the relationship between spine structure and function. The advent of multi-photon laser-scanning microscopy has greatly improved our ability to investigate the dynamic interplay between spine form and function. Regulated structural changes occur at spines undergoing plasticity, offering a mechanism to account for the well-described correlation between spine size and synapse strength. In turn, spine structure can influence the degree of biochemical and perhaps electrical compartmentalization at individual synapses. Here, we review the relationship between dendritic spine morphology, features of spine compartmentalization and synaptic plasticity. We highlight emerging molecular mechanisms that link structural and functional changes in spines during plasticity, and also consider circumstances that underscore some divergence from a tight structure-function coupling. Because of the intricate influence of spine structure on biochemical and electrical signalling, activity-dependent changes in spine morphology alone may thus contribute to the metaplastic potential of synapses. This possibility asserts a role for structural dynamics in neuronal information storage and aligns well with current computational models.
Figures
References
-
- Cajal SR. Estructura de los centros nerviosos de las aves. Revista Trimestral de Histología Normal y Patológica. 1888;1:1–10.
-
- Yuste R. Dendritic Spines. Cambridge, Mass, USA: The MIT Press; 2010.
-
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

