Mechanisms of myocardial regeneration
- PMID: 22578241
- PMCID: PMC3356689
- DOI: 10.1016/j.tcm.2012.02.006
Mechanisms of myocardial regeneration
Abstract
Traditionally, the adult heart has been viewed as a terminally differentiated postmitotic organ in which the number of cardiomyocytes is established at birth and these cells persist throughout the life span of the organ and organism. However, the discovery that cardiac stem cells live in the heart and differentiate into the various cardiac cell lineages has dramatically changed our understanding of myocardial biology. Deciphering the biological processes that lead to myocyte renewal is a challenging task. Cardiac regeneration may be accomplished by (1) commitment of multipotent stem cells that generate all specialized lineages within the parenchyma, (2) activation of unipotent progenitors with restricted differentiation potential, (3) replication of pre-existing differentiated cells, (4) transdifferentiation of exogenous progenitors that undergo plastic conversion into cells different from the organ of origin, and (5) dedifferentiation of cardiomyocytes that re-enter the cell cycle and divide. These multiple mechanisms of cell growth may act in concert to regenerate complex structures and restore the function of the target organ.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
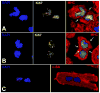
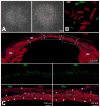
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- R01 HL114346/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 AG026107/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

