Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development
- PMID: 22579044
- PMCID: PMC3383994
- DOI: 10.1016/j.cell.2012.04.011
Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development
Abstract
The presence of ribonucleotides in genomic DNA is undesirable given their increased susceptibility to hydrolysis. Ribonuclease (RNase) H enzymes that recognize and process such embedded ribonucleotides are present in all domains of life. However, in unicellular organisms such as budding yeast, they are not required for viability or even efficient cellular proliferation, while in humans, RNase H2 hypomorphic mutations cause the neuroinflammatory disorder Aicardi-Goutières syndrome. Here, we report that RNase H2 is an essential enzyme in mice, required for embryonic growth from gastrulation onward. RNase H2 null embryos accumulate large numbers of single (or di-) ribonucleotides embedded in their genomic DNA (>1,000,000 per cell), resulting in genome instability and a p53-dependent DNA-damage response. Our findings establish RNase H2 as a key mammalian genome surveillance enzyme required for ribonucleotide removal and demonstrate that ribonucleotides are the most commonly occurring endogenous nucleotide base lesion in replicating cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

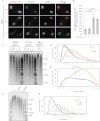
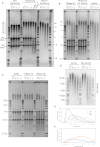
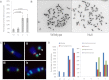
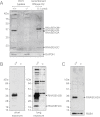

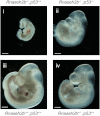
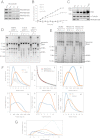
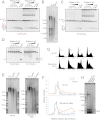
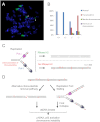
References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Garland Science Taylor & Francis Group; New York: 2002. Molecular biology of the Cell.
-
- Arudchandran A., Cerritelli S., Narimatsu S., Itaya M., Shin D.Y., Shimada Y., Crouch R.J. The absence of ribonuclease H1 or H2 alters the sensitivity of Saccharomyces cerevisiae to hydroxyurea, caffeine and ethyl methanesulphonate: implications for roles of RNases H in DNA replication and repair. Genes Cells. 2000;5:789–802. - PubMed
-
- Bhoj V.G., Chen Z.J. Linking retroelements to autoimmunity. Cell. 2008;134:569–571. - PubMed
-
- Bogenhagen D., Clayton D.A. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell. 1977;11:719–727. - PubMed
Supplemental References
-
- Adam, J., Deans, B., and Thacker, J. (2007). A role for Xrcc2 in the early stages of mouse development. DNA Repair (Amst.) 6, 224–234. - PubMed
-
- Armstrong, J.F., Kaufman, M.H., Harrison, D.J., and Clarke, A.R. (1995). High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 5, 931–936. - PubMed
-
- Bouwman, P., Drost, R., Klijn, C., Pieterse, M., van der Gulden, H., Song, J.Y., Szuhai, K., and Jonkers, J. (2011). Loss of p53 partially rescues embryonic development of Palb2 knockout mice but does not foster haploinsufficiency of Palb2 in tumour suppression. J. Pathol. 224, 10–21. - PubMed
-
- Dunning, M.J., Smith, M.L., Ritchie, M.E., and Tavaré, S. (2007). beadarray: R classes and methods for Illumina bead-based data. Bioinformatics 23, 2183–2184. - PubMed
-
- Hakem, R., de la Pompa, J.L., Elia, A., Potter, J., and Mak, T.W. (1997). Partial rescue of Brca1 (5-6) early embryonic lethality by p53 or p21 null mutation. Nat. Genet. 16, 298–302. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- MC_U105663140/MRC_/Medical Research Council/United Kingdom
- MC_U127584494/MRC_/Medical Research Council/United Kingdom
- MC_PC_U127597124/MRC_/Medical Research Council/United Kingdom
- MC_PC_U127580972/MRC_/Medical Research Council/United Kingdom
- MC_U127597124/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

