Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling
- PMID: 22579286
- PMCID: PMC3371233
- DOI: 10.1016/j.cell.2012.03.032
Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling
Erratum in
- Cell. 2012 Oct 26;151(3):687-9
Abstract
Localized protein synthesis requires assembly and transport of translationally silenced ribonucleoprotein particles (RNPs), some of which are exceptionally large. Where in the cell such large RNP granules first assemble was heretofore unknown. We previously reported that during synapse development, a fragment of the Wnt-1 receptor, DFrizzled2, enters postsynaptic nuclei where it forms prominent foci. Here we show that these foci constitute large RNP granules harboring synaptic protein transcripts. These granules exit the nucleus by budding through the inner and the outer nuclear membranes in a nuclear egress mechanism akin to that of herpes viruses. This budding involves phosphorylation of A-type lamin, a protein linked to muscular dystrophies. Thus nuclear envelope budding is an endogenous nuclear export pathway for large RNP granules.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
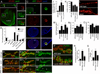
D,E- a wild type NMJ at (A) low and (B) high magnification,
F, G- a lamC null mutant NMJ at (C) low and (D) high magnification, and
H, I- an NMJ from a larva expressing LamC-RNAi in muscles (LamC-RNAi-muscle).
J- ghost boutons, and
K- bouton number.
M- mEJP frequency,
N- mEJPs amplitude, and
O- evoked EJP amplitude.
(P) wild type and
(Q) a larva expressing LamC-RNAi in muscles.
(R) GluRIIA label volume and
(S) total intensity.
***= p< 0.0001; **= p<0.01; *= p<0.05. Bars in graphs indicate mean±SEM.
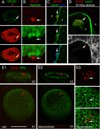
A, B- propidium iodide and Hoechst,
C- the membrane marker Concanavalin-A (ConA; deconvolved),
D- 70 KDa dextran injected into the cytoplasm (deconvolved),
E- mAb414 labeling the nuclear pore complex (NPC;(Davis and Blobel, 1986)).
E1 shows a raw image of the nucleus,
E2 a deconvolution of E1, and
E3 a high magnification view of the focus in E2.
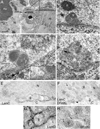
B- larval muscle.
C, D- S2 cells. Dense granules appear to be bounded by membrane (arrow in D).
E, G- anti-LamC and 18nm gold-conjugated second antibody shown at (E) low and (G) high magnification;
F, H- anti-DFz2C and anti-LamC with 12nm and 18nm gold conjugated second antibody, respectively, shown at (F) low and (G) high magnification.

C- without or
D- with RNase treatment.
E- treated with rEDTA and
F- not treated with EDTA (same preparation as in (E)). N= nucleus; C= cytoplasm.
E2, F2- High magnification views at the nuclear area of E1 and F1 around a DFz2C granule showing a dark meshwork surrounding the granule, which is bleached after rEDTA treatment (E2), while DFz2C granules retain electron density (F2).
E3, F3- High magnification of E1 and F1, showing ribosomes in the cytoplasm, which retain electron density after EDTA treatment.
G- Low magnification view of a muscle focus showing retention of electron density by DFz2C granules after rEDTA (g; arrow).
Arrowheads=ribosomes at ONM. Arrow=cytoplasmic granule of the same size and morphology as DFz2C granules at INM invaginations.
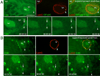
Top rows in A, B show (top panel) a single image of a larval body wall muscle nucleus labeled with E36, (middle panel) the same nucleus after fixation and immunolabeling with DFz2C and/or LamC antibodies, and (right panel) their superposition obtained after resizing using fiduciary markers. Arrowheads=position of the E36 and DFz2C/LamC foci.
Bottom rows in A, B display time-lapse imaging series, showing an E36 labeled granule exiting the nucleus of a larval body wall muscle. Arrows = initial position of the granule; arrowheads= granules while moving away from the nucleus. Time marks=hr:min:sec.
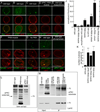
A- localization of Baz at LamC foci,
B- diffuse distribution of aPKC in muscle nuclei,
C- a DFz2C/LamC foci in wild type muscle nucleus,
D- virtual elimination of DFz2C/LamC foci by expressing aPKC-RNAi in muscles,
E- that chelerythrine feeding, decreases DFz2C/LamC foci,
F- that expressing PKM in muscles enlarges and increases LamC foci number, which are devoid of DFz2C,
G- that expressing PKM for just 30 min increases DFz2C/lamC foci number,
H- enrichment of PKC phosphorylated substrates immunoreactivity at LamC foci, and
I- that this label is eliminated after treatment with lambda phosphatase.
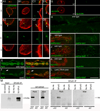
A- association of par6 mRNA with a LamC focus (arrow);
B- localization of par6 transcript near a nuclear membrane fold;
C, D- localization of par6 transcript to cytoplasmically directed projections of the nuclear boundary.
A2–D2 are high magnification views of the nuclei shown in A1–D1.
G- Absence of FISH signal when using a wg probe;
H- a low magnification view of par6 mRNA at the postsynaptic larval NMJ;
I–J synaptic par6 mRNA localization using 2 different par6 probes;
K- absence of synaptic wg mRNA localization;
L- synaptic par6 mRNA localization in a larva expressing LamC-RNAi in muscle, showing virtual elimination of postsynaptic par6 mRNA.
E- wild type and
F- lamC mutant showing a ghost bouton (arrow) devoid of Par6 immunoreactivity and a general decrease in Par6 levels throughout the NMJ.
Comment in
-
RNP export by nuclear envelope budding.Cell. 2012 May 11;149(4):733-5. doi: 10.1016/j.cell.2012.04.018. Cell. 2012. PMID: 22579277
-
Nuclear transport: A new way out.Nat Rev Mol Cell Biol. 2012 Jun 8;13(7):407. doi: 10.1038/nrm3378. Nat Rev Mol Cell Biol. 2012. PMID: 22678484 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

