Neuroendocrine aspects of catamenial epilepsy
- PMID: 22579656
- PMCID: PMC3422425
- DOI: 10.1016/j.yhbeh.2012.04.016
Neuroendocrine aspects of catamenial epilepsy
Abstract
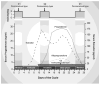
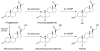
This review describes the neuroendocrinological aspects of catamenial epilepsy, a menstrual cycle-related seizure disorder in women with epilepsy. Catamenial epilepsy is a multifaceted neuroendocrine condition in which seizures are clustered around specific points in the menstrual cycle, most often around perimenstrual or periovulatory period. Three types of catamenial seizures (perimenstrual, periovulatory and inadequate luteal) have been identified. The molecular pathophysiology of catamenial epilepsy remains unclear. Cyclical changes in the circulating levels of estrogens and progesterone (P) play a central role in the development of catamenial epilepsy. Endogenous neurosteroids such as allopregnanolone (AP) and allotetrahydrodeoxycorticosterone (THDOC) that modulate seizure susceptibility could play a critical role in catamenial epilepsy. In addition, plasticity in GABA-A receptor subunits could play a role in the enhanced seizure susceptibility in catamenial epilepsy. P-derived neurosteroids such as AP and THDOC potentiate synaptic GABA-A receptor function and also activate extrasynaptic GABA-A receptors in the hippocampus and thus may represent endogenous regulators of catamenial seizure susceptibility. Experimental studies have shown that neurosteroids confer greater seizure protection in animal models of catamenial epilepsy, especially without evident tolerance to their actions during chronic therapy. In the recently completed NIH-sponsored, placebo controlled phase 3 clinical trial, P therapy proved to be beneficial only in women with perimenstrual catamenial epilepsy but not in non-catamenial subjects. Neurosteroid analogs with favorable profile may be useful in the treatment of catamenial epilepsy.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abassi F, Krumholz A, Kittner SJ, Langenberg P. Effects of menopause on seizures in women with epilepsy. Epilepsia. 1999;42:205–210. - PubMed
-
- Amado D, Cavalheiro EA. Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 1998;32:266–274. - PubMed
-
- Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. - PubMed
-
- Bäckström T, Zetterlund B, Blom S, Romano M. Effect of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

