Lateral interactions in the outer retina
- PMID: 22580106
- PMCID: PMC3401171
- DOI: 10.1016/j.preteyeres.2012.04.003
Lateral interactions in the outer retina
Abstract
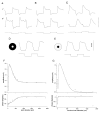
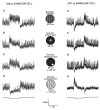
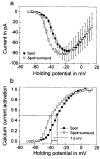
Lateral interactions in the outer retina, particularly negative feedback from horizontal cells to cones and direct feed-forward input from horizontal cells to bipolar cells, play a number of important roles in early visual processing, such as generating center-surround receptive fields that enhance spatial discrimination. These circuits may also contribute to post-receptoral light adaptation and the generation of color opponency. In this review, we examine the contributions of horizontal cell feedback and feed-forward pathways to early visual processing. We begin by reviewing the properties of bipolar cell receptive fields, especially with respect to modulation of the bipolar receptive field surround by the ambient light level and to the contribution of horizontal cells to the surround. We then review evidence for and against three proposed mechanisms for negative feedback from horizontal cells to cones: 1) GABA release by horizontal cells, 2) ephaptic modulation of the cone pedicle membrane potential generated by currents flowing through hemigap junctions in horizontal cell dendrites, and 3) modulation of cone calcium currents (I(Ca)) by changes in synaptic cleft proton levels. We also consider evidence for the presence of direct horizontal cell feed-forward input to bipolar cells and discuss a possible role for GABA at this synapse. We summarize proposed functions of horizontal cell feedback and feed-forward pathways. Finally, we examine the mechanisms and functions of two other forms of lateral interaction in the outer retina: negative feedback from horizontal cells to rods and positive feedback from horizontal cells to cones.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures








References
-
- Agardh E, Bruun A, Ehinger B, Storm-Mathisen J. GABA immunoreactivity in the retina. Invest Ophthalmol Vis Sci. 1986;27:674–678. - PubMed
-
- Agardh E, Ehinger B, Wu JY. GABA and GAD-like immunoreactivity in the primate retina. Histochem. 1987;86:485–490. - PubMed
-
- Ammermüller J, Muller JF, Kolb H. The organization of the turtle inner retina. II Analysis of color-coded and directionally selective cells. J Comp Neurol. 1995;358:35–62. - PubMed
-
- Arvanitaki A. Effects evoked in an axon by activity of a contiguous one. J Neurophysiol. 1942;5:89–108.
-
- Asi H, Perlman I. Neural interactions between cone photoreceptors and horizontal cells in the turtle (Mauremys caspica) retina. Vis Neurosci. 1998;15:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

