MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches
- PMID: 22580612
- PMCID: PMC3621754
- DOI: 10.1038/onc.2012.158
MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches
Abstract
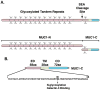
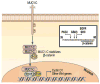
Mucin 1 (MUC1) is a heterodimeric protein formed by two subunits that is aberrantly overexpressed in human breast cancer and other cancers. Historically, much of the early work on MUC1 focused on the shed mucin subunit. However, more recent studies have been directed at the transmembrane MUC1-C-terminal subunit (MUC1-C) that functions as an oncoprotein. MUC1-C interacts with EGFR (epidermal growth factor receptor), ErbB2 and other receptor tyrosine kinases at the cell membrane and contributes to activation of the PI3KAKT and mitogen-activated protein kinase kinase (MEK)extracellular signal-regulated kinase (ERK) pathways. MUC1-C also localizes to the nucleus where it activates the Wnt/β-catenin, signal transducer and activator of transcription (STAT) and NF (nuclear factor)-κB RelA pathways. These findings and the demonstration that MUC1-C is a druggable target have provided the experimental basis for designing agents that block MUC1-C function. Notably, inhibitors of the MUC1-C subunit have been developed that directly block its oncogenic function and induce death of breast cancer cells in vitro and in xenograft models. On the basis of these findings, a first-in-class MUC1-C inhibitor has entered phase I evaluation as a potential agent for the treatment of patients with breast cancers who express this oncoprotein.
Conflict of interest statement
Figures




References
-
- Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. - PubMed
-
- Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992;267:6171–7. - PubMed
-
- Levitin F, Stern O, Weiss M, Gil-Henn C, Ziv R, Prokocimer Z, et al. The MUC1 SEA module is a self-cleaving domain. J Biol Chem. 2005;280:33374–86. - PubMed
-
- Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol. 2006;13:71–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

