3'-processing and strand transfer catalysed by retroviral integrase in crystallo
- PMID: 22580823
- PMCID: PMC3395085
- DOI: 10.1038/emboj.2012.118
3'-processing and strand transfer catalysed by retroviral integrase in crystallo
Abstract
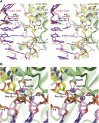
Retroviral integrase (IN) is responsible for two consecutive reactions, which lead to insertion of a viral DNA copy into a host cell chromosome. Initially, the enzyme removes di- or trinucleotides from viral DNA ends to expose 3'-hydroxyls attached to the invariant CA dinucleotides (3'-processing reaction). Second, it inserts the processed 3'-viral DNA ends into host chromosomal DNA (strand transfer). Herein, we report a crystal structure of prototype foamy virus IN bound to viral DNA prior to 3'-processing. Furthermore, taking advantage of its dependence on divalent metal ion cofactors, we were able to freeze trap the viral enzyme in its ground states containing all the components necessary for 3'-processing or strand transfer. Our results shed light on the mechanics of retroviral DNA integration and explain why HIV IN strand transfer inhibitors are ineffective against the 3'-processing step of integration. The ground state structures moreover highlight a striking substrate mimicry utilized by the inhibitors in their binding to the IN active site and suggest ways to improve upon this clinically relevant class of small molecules.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Brautigam CA, Steitz TA (1998) Structural principles for the inhibition of the 3′–5′ exonuclease activity of Escherichia coli DNA polymerase I by phosphorothioates. J Mol Biol 277: 363–377 - PubMed
-
- Cherepanov P, Maertens GN, Hare S (2011) Structural insights into the retroviral DNA integration apparatus. Curr Opin Struct Biol 21: 249–256 - PubMed
-
- Craigie R (2002) Retroviral DNA Integration. InMobile DNA II Craig NL, Craigie R, Gellert M, Lambowitz AM (eds), pp613–630. Washington DC: ASM Press
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

