Channel properties reveal differential expression of TARPed and TARPless AMPARs in stargazer neurons
- PMID: 22581185
- PMCID: PMC3427011
- DOI: 10.1038/nn.3107
Channel properties reveal differential expression of TARPed and TARPless AMPARs in stargazer neurons
Abstract
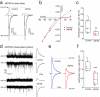
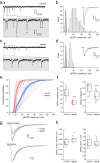
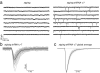
Dynamic regulation of calcium-permeable AMPA receptors (CP-AMPARs) is important for normal synaptic transmission, plasticity and pathological changes. Although the involvement of transmembrane AMPAR regulatory proteins (TARPs) in trafficking of calcium-impermeable AMPARs (CI-AMPARs) has been extensively studied, their role in the surface expression and function of CP-AMPARs remains unclear. We examined AMPAR-mediated currents in cerebellar stellate cells from stargazer mice, which lack the prototypical TARP stargazin (g-2). We found a marked increase in the contribution of CP-AMPARs to synaptic responses, indicating that, unlike CI-AMPARs, these can localize at synapses in the absence of g-2. In contrast with CP-AMPARs in extrasynaptic regions, synaptic CP-AMPARs displayed an unexpectedly low channel conductance and strong block by intracellular spermine, suggesting that they were ‘TARPless’. As a proof of principle that TARP association is not an absolute requirement for AMPAR clustering at synapses, miniature excitatory postsynaptic currents mediated by TARPless AMPARs were readily detected in stargazer granule cells following knockdown of their only other TARP, g-7.
Figures





References
-
- Chen L, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

