Direct evidence that HSV DNA damaged by ultraviolet (UV) irradiation can be repaired in a cell type-dependent manner
- PMID: 22581427
- PMCID: PMC3361605
- DOI: 10.1007/s13365-012-0105-2
Direct evidence that HSV DNA damaged by ultraviolet (UV) irradiation can be repaired in a cell type-dependent manner
Abstract
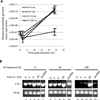
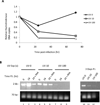
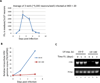
Infection of permissive cells, in tissue culture, with herpes simplex virus (HSV) has been reported to induce host DNA damage repair responses that are necessary for efficient viral replication. However, direct repair of the damaged viral DNA has not, to our knowledge, been shown. In this report, we detect and determine the amount of damaged HSV-1 DNA, following introduction of experimentally damaged HSV genomes into tissue cultures of permissive Vero, NGF differentiated PC12 cells and primary rat neurons, using a method of detection introduced here. The results show that HSV-1 strain 17 DNA containing UV-induced DNA damage is efficiently repaired, in Vero, but not NGF differentiated PC12 cells. The primary rat neuronal cultures were capable of repairing the damaged viral DNA, but with much less efficiency than did the permissive Vero cells. Moreover, by conducting the experiments with either an inhibitor of the HSV polymerase (phosphonoacetic acid [PAA]) or with a replication defective DNA polymerase mutant virus, HP66, the results suggest that repair can occur in the absence of a functional viral polymerase, although polymerase function seems to enhance the efficiency of the repair, in a replication independent manner. The possible significance of varying cell type mediated repair of viral DNA to viral pathogenesis is discussed.
Figures



References
-
- Block T, Barney S, Masonis J, Maggioncalda J, Valyi-Nagy T, Fraser NW. Long term herpes simplex virus type 1 infection of nerve growth factor-treated PC12 cells. J Gen Virol. 1994;75(Pt 9):2481–2487. - PubMed
-
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. - PubMed
-
- Danaher RJ, Jacob RJ, Chorak MD, Freeman CS, Miller CS. Heat stress activates production of herpes simplex virus type 1 from quiescently infected neurally differentiated PC12 cells. J Neurovirol. 1999;5:374–383. - PubMed
-
- Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don't divide what's to repair? Mutat Res. 2007;614:24–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

