Systems pharmacology, pharmacogenetics, and clinical trial design in network medicine
- PMID: 22581565
- PMCID: PMC3783940
- DOI: 10.1002/wsbm.1173
Systems pharmacology, pharmacogenetics, and clinical trial design in network medicine
Abstract
The rapidly growing disciplines of systems biology and network science are now poised to meet the fields of clinical medicine and pharmacology. Principles of systems pharmacology can be applied to drug design and, ultimately, testing in human clinical trials. Rather than focusing exclusively on single drug targets, systems pharmacology examines the holistic response of a phenotype-dependent pathway or pathways to drug perturbation. Knowledge of individual pharmacogenetic profiles further modulates the responses to these drug perturbations, moving the field toward more individualized ('personalized') drug development. The speed with which the information required to assess these system responses and their genomic underpinnings is changing and the importance of identifying the optimal drug or drug combinations for maximal benefit and minimal risk require that clinical trial design strategies be adaptable. In this paper, we review the tenets of adaptive clinical trial design as they may apply to an era of expanding knowledge of systems pharmacology and pharmacogenomics, and clinical trail design in network medicine.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


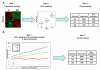




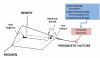

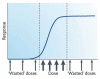

References
-
- Lindsay MA. Target discovery. Nat Rev Drug Discov. 2003;2(10):831–838. - PubMed
-
- Alexander JC, Salazar DE. Modern drug discovery and development. In: Robertson D, editor. Clinical and Translational Science: Principles of Human Research. Academic Press; London: 2009. pp. 361–380.
-
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–185. - PubMed
-
- Adams CP, Brantner VV. Spending on new drug development1. Health Econ. 2010;19(2):130–141. - PubMed
-
- Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477(7366):526–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL108630/HL/NHLBI NIH HHS/United States
- U54 HL070819/HL/NHLBI NIH HHS/United States
- P01 HL048743/HL/NHLBI NIH HHS/United States
- R01 HL061795/HL/NHLBI NIH HHS/United States
- U01 HL065899/HL/NHLBI NIH HHS/United States
- HL108630/HL/NHLBI NIH HHS/United States
- HL48743/HL/NHLBI NIH HHS/United States
- R37 HL061795/HL/NHLBI NIH HHS/United States
- HL107192/HL/NHLBI NIH HHS/United States
- HL70819/HL/NHLBI NIH HHS/United States
- P50 HL107192/HL/NHLBI NIH HHS/United States
- HL0655899/HL/NHLBI NIH HHS/United States
- HL61795/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

