Physicochemical properties of epidermal growth factor receptor inhibitors and development of a nanoliposomal formulation of gefitinib
- PMID: 22581704
- PMCID: PMC4131431
- DOI: 10.1002/jps.23180
Physicochemical properties of epidermal growth factor receptor inhibitors and development of a nanoliposomal formulation of gefitinib
Abstract
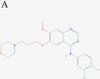
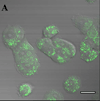
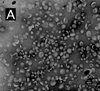
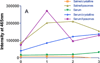
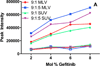
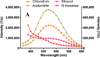
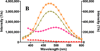
Inhibitors of epidermal growth factor (EGF) receptor (EGFR) tyrosine kinases show efficacy in cancers that are highly addicted to nonmutated EGF signaling, but off-target effects limit therapy. Carrier-based formulations could reduce drug deposition in normal tissues, enhance tumor deposition, and reduce free drug concentrations, thereby reducing the side effects. Therefore, the feasibility of developing nanoliposomal formulations of EGFR inhibitors was investigated. Gefitinib and erlotinib fluorescence was characterized as a tool for formulation development. Peak excitation was 345 nm and peak emission was 385-465 nm, depending upon the environment polarity. Emission was negligible in water but intense in nonpolar solvents, membranes, or bound to serum proteins. Cellular uptake and distribution could also be imaged by fluorescence in drug-resistant tumor spheroids. Gefitinib fluorescence characteristics enabled facile optimization of formulations. Although 4-6 mol % gefitinib could be incorporated in the liposome bilayer, 40-60 mol % could be encapsulated in stable, remote-loaded liposomes consisting of distearoylphosphatidylcholine-polyethylene glycol-distereoylphosphatidylethanolamine-cholesterol (9:1:5 mol:mol:mol). Drug leakage in serum, monitored by fluorescence, was minimal over 24 h at 37°C. The results provide both promising lead formulations as well as novel tools for evaluating new formulations of structurally similar receptor tyrosine kinase inhibitors and their cellular uptake and tissue biodistribution.
Copyright © 2012 Wiley Periodicals, Inc.
Figures









Similar articles
-
Novel liposomal gefitinib (L-GEF) formulations.Anticancer Res. 2012 Jul;32(7):2919-23. Anticancer Res. 2012. PMID: 22753756
-
Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients.Cancer Biol Ther. 2007 Mar;6(3):432-8. doi: 10.4161/cbt.6.3.3763. Epub 2007 Mar 29. Cancer Biol Ther. 2007. PMID: 17312388
-
Gefitinib for non-small cell lung cancer patients with liver cirrhosis.Intern Med. 2009;48(18):1677-9. doi: 10.2169/internalmedicine.48.2401. Epub 2009 Sep 15. Intern Med. 2009. PMID: 19755773
-
Clinical pharmacokinetics of tyrosine kinase inhibitors: focus on 4-anilinoquinazolines.Clin Pharmacokinet. 2011 Jun;50(6):371-403. doi: 10.2165/11587020-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21553932 Review.
-
[Anti-EGFR therapy: towards a "à la carte" targeting of hepatocellular carcinoma].Gastroenterol Clin Biol. 2006 Oct;30(10):1133-5. doi: 10.1016/s0399-8320(06)73490-x. Gastroenterol Clin Biol. 2006. PMID: 17075466 Review. French. No abstract available.
Cited by
-
Development of a nanoliposomal formulation of erlotinib for lung cancer and in vitro/in vivo antitumoral evaluation.Drug Des Devel Ther. 2017 Dec 18;12:1-8. doi: 10.2147/DDDT.S146925. eCollection 2018. Drug Des Devel Ther. 2017. Retraction in: Drug Des Devel Ther. 2022 Nov 18;16:4019-4020. doi: 10.2147/DDDT.S398024. PMID: 29296076 Free PMC article. Retracted.
-
In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer.Drug Deliv. 2017 Nov;24(1):1501-1512. doi: 10.1080/10717544.2017.1384862. Drug Deliv. 2017. PMID: 28961023 Free PMC article.
-
Pulmonary delivery of osimertinib liposomes for non-small cell lung cancer treatment: formulation development and in vitro evaluation.Drug Deliv Transl Res. 2022 Oct;12(10):2474-2487. doi: 10.1007/s13346-021-01088-0. Epub 2021 Nov 23. Drug Deliv Transl Res. 2022. PMID: 34816394
-
Development of Liposomal Vesicles for Osimertinib Delivery to EGFR Mutation-Positive Lung Cancer Cells.Pharmaceutics. 2020 Sep 30;12(10):939. doi: 10.3390/pharmaceutics12100939. Pharmaceutics. 2020. PMID: 33008019 Free PMC article.
-
Intrinsically Fluorescent Anti-Cancer Drugs.Biology (Basel). 2022 Jul 28;11(8):1135. doi: 10.3390/biology11081135. Biology (Basel). 2022. PMID: 36009762 Free PMC article. Review.
References
-
- Rocha-Lima CM, Soares HP, Raez LE, Singal R. EGFR targeting of solid tumors. Cancer Control. 2007;14(3):295–304. - PubMed
-
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62(20):5749–5754. - PubMed
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. - PubMed
-
- Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R, Wang Y, MacNeill J, Mitchell J, Gygi SP, Rush J, Polakiewicz RD, Comb MJ. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105(2):692–697. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

