The FleQ protein from Pseudomonas aeruginosa functions as both a repressor and an activator to control gene expression from the pel operon promoter in response to c-di-GMP
- PMID: 22581773
- PMCID: PMC3424551
- DOI: 10.1093/nar/gks384
The FleQ protein from Pseudomonas aeruginosa functions as both a repressor and an activator to control gene expression from the pel operon promoter in response to c-di-GMP
Abstract
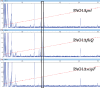
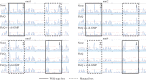
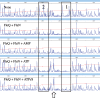
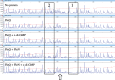
Bis-(3'-5')-cyclic dimeric guanosine monophosphate (c-di-GMP) modulates the transition between planktonic and biofilm life styles. In response to c-di-GMP, the enhancer binding protein FleQ from Pseudomonas aeruginosa derepresses the expression of Pel exopolysaccharide genes required for biofilm formation when a second protein, FleN is present. A model is that binding of c-di-GMP to FleQ induces its dissociation from the pelA promoter allowing RNA polymerase to access this site. To test this, we analyzed pelA DNA footprinting patterns with various combinations of FleQ, FleN and c-di-GMP, coupled to in vivo promoter activities. FleQ binds to two sites called box 1 and 2. FleN binds to FleQ bound at these sites causing the intervening DNA to bend. Binding of c-di-GMP to FleQ relieves the DNA distortion but FleQ remains bound to the two sites. Analysis of wild type and mutated versions of pelA-lacZ transcriptional fusions suggests that FleQ represses gene expression from box 2 and activates gene expression in response to c-di-GMP from box 1. The role of c-di-GMP is thus to convert FleQ from a repressor to an activator. The mechanism of action of FleQ is distinct from that of other bacterial transcription factors that both activate and repress gene expression from a single promoter.
Figures





References
-
- Simm R, Morr M, Kader A, Nimtz M, Romling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004;53:1123–1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

