Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2
- PMID: 22581805
- PMCID: PMC3382349
- DOI: 10.1093/glycob/cws083
Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2
Abstract
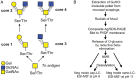
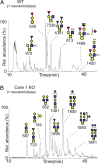
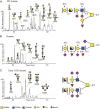
The heavily O-glycosylated mucin MUC2 constitutes the major protein in the mucosal layer that acts as a physical barrier protecting the epithelial layer in the colon. In this study, Muc2 was purified from mucosal scrapings from the colon of wild-type (WT) mice, core 3 transferase knockout (C3Gnt(-/-)) mice and intestinal epithelial cell-specific core 1 knockout (IEC C1Galt1(-/-)) mice. The Muc2 O-glycans were released by reductive β-elimination and analyzed with liquid chromatography-mass spectrometry in the negative-ion mode. Muc2 from the distal colon of WT and C3Gnt(-/-) knockout mice carried a mixture of core 1- or core 2-type glycans, whereas Muc2 from IEC C1Galt1(-/-) mice carried highly sialylated core 3- and core 4-type glycans. A large portion of NeuAc in all mouse models was positioned on disialylated N-acetyllactosamine units, an epitope not reported on human colonic MUC2. Mass spectra and proton NMR spectroscopy revealed an abundant NeuAc linked to internally positioned N-acetylglucosamine on colonic murine Muc2, which also differs markedly from human MUC2. Our results highlight that murine colonic Muc2 O-glycosylation is substantially different from human MUC2, which could be one explanation for the different commensal microbiota of these two species.
Figures



Similar articles
-
Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution.Am J Physiol Gastrointest Liver Physiol. 2013 Sep 1;305(5):G357-63. doi: 10.1152/ajpgi.00048.2013. Epub 2013 Jul 5. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23832516 Free PMC article.
-
Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice.Gastroenterology. 2016 Jul;151(1):152-164.e11. doi: 10.1053/j.gastro.2016.03.039. Epub 2016 Apr 6. Gastroenterology. 2016. PMID: 27059389 Free PMC article.
-
The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis.Infect Immun. 2013 Oct;81(10):3672-83. doi: 10.1128/IAI.00854-13. Epub 2013 Jul 22. Infect Immun. 2013. PMID: 23876803 Free PMC article.
-
The Densely O-Glycosylated MUC2 Mucin Protects the Intestine and Provides Food for the Commensal Bacteria.J Mol Biol. 2016 Aug 14;428(16):3221-3229. doi: 10.1016/j.jmb.2016.02.010. Epub 2016 Feb 12. J Mol Biol. 2016. PMID: 26880333 Free PMC article. Review.
-
Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer.Biol Pharm Bull. 2012;35(10):1637-41. doi: 10.1248/bpb.b12-00412. Biol Pharm Bull. 2012. PMID: 23037153 Review.
Cited by
-
Identification by mass spectrometry and immunoblotting of xenogeneic antigens in the N- and O-glycomes of porcine, bovine and equine heart tissues.Glycoconj J. 2020 Aug;37(4):485-498. doi: 10.1007/s10719-020-09931-1. Epub 2020 Jun 15. Glycoconj J. 2020. PMID: 32542517 Free PMC article.
-
Revealing Glycosylation Patterns in In Vitro-Produced Mucus Exposed to Pasteurized Mucus-Associated Intestinal Microbes by MALDI-TOF-MS and PGC-LC-MS/MS.J Agric Food Chem. 2024 Jul 10;72(27):15345-15356. doi: 10.1021/acs.jafc.4c01401. Epub 2024 Jun 26. J Agric Food Chem. 2024. PMID: 38932522 Free PMC article.
-
Altered mucus glycosylation in core 1 O-glycan-deficient mice affects microbiota composition and intestinal architecture.PLoS One. 2014 Jan 9;9(1):e85254. doi: 10.1371/journal.pone.0085254. eCollection 2014. PLoS One. 2014. PMID: 24416370 Free PMC article.
-
Fight them or feed them: how the intestinal mucus layer manages the gut microbiota.Gastroenterol Rep (Oxf). 2019 Feb;7(1):3-12. doi: 10.1093/gastro/goy052. Epub 2019 Feb 13. Gastroenterol Rep (Oxf). 2019. PMID: 30792861 Free PMC article. Review.
-
Spatially distinct physiology of Bacteroides fragilis within the proximal colon of gnotobiotic mice.Nat Microbiol. 2020 May;5(5):746-756. doi: 10.1038/s41564-020-0683-3. Epub 2020 Mar 9. Nat Microbiol. 2020. PMID: 32152589 Free PMC article.
References
-
- Andersch-Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–716. - PubMed
-
- Axelsson MA, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–18870. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

