Rituximab in children with resistant idiopathic nephrotic syndrome
- PMID: 22581994
- PMCID: PMC3358759
- DOI: 10.1681/ASN.2011080775
Rituximab in children with resistant idiopathic nephrotic syndrome
Abstract
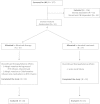
Idiopathic nephrotic syndrome resistant to standard treatments remains a therapeutic dilemma in pediatric nephrology. To test whether the anti-CD20 monoclonal antibody rituximab may benefit these patients, we conducted an open-label, randomized, controlled trial in 31 children with idiopathic nephrotic syndrome unresponsive to the combination of calcineurin inhibitors and prednisone. All children continued prednisone and calcineurin inhibitors at the doses prescribed before enrollment, and one treatment group received two doses of rituximab (375 mg/m(2) intravenously) as add-on therapy. The mean age was 8 years (range, 2-16 years). Rituximab did not reduce proteinuria at 3 months (change, -12% [95% confidence interval, -73% to 110%]; P=0.77 in analysis of covariance model adjusted for baseline proteinuria). Additional adjustment for previous remission and interaction terms (treatment by baseline proteinuria and treatment by previous remission) did not change the results. In conclusion, these data do not support the addition of rituximab to prednisone and calcineurin inhibitors in children with resistant idiopathic nephrotic syndrome.
Figures

Comment in
-
Rituximab in steroid-resistant nephrotic syndrome in children: a (false) glimmer of hope?J Am Soc Nephrol. 2012 Jun;23(6):975-8. doi: 10.1681/ASN.2012040413. Epub 2012 May 10. J Am Soc Nephrol. 2012. PMID: 22581992 No abstract available.
References
-
- Cohen AH, Border WA, Glassock RJ: Nehprotic syndrome with glomerular mesangial IgM deposits. Lab Invest 38: 610–619, 1978 - PubMed
-
- Korbet SM: Primary focal segmental glomerulosclerosis. J Am Soc Nephrol 9: 1333–1340, 1998 - PubMed
-
- McAdams AJ, Valentini RP, Welch TR: The nonspecificity of focal segmental glomerulosclerosis. The defining characteristics of primary focal glomerulosclerosis, mesangial proliferation, and minimal change. Medicine (Baltimore) 76: 42–52, 1997 - PubMed
-
- Tryggvason K, Wartiovaara J: Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10: 543–549, 2001 - PubMed
-
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

