Evidence for Purinergic Receptors in Vestibular Dark Cell and Strial Marginal Cell Epithelia of Gerbil
- PMID: 22582019
- PMCID: PMC3348583
Evidence for Purinergic Receptors in Vestibular Dark Cell and Strial Marginal Cell Epithelia of Gerbil
Abstract
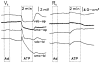
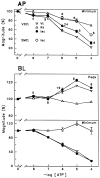
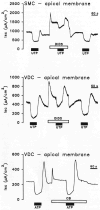
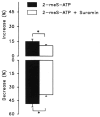
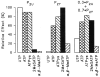
Purinergic receptors have been found to modulate ion transport in several types of epithelial cells as well as excitable cells. It was of interest to determine whether vestibular dark cells and strial marginal cells contain purinergic receptors in either the apicalor basolateral membrane which modulate transepithelial ion transport. Vestibular dark cell and strial marginal cell epithelia were mounted in a micro-Ussing chamber for the measurement of the transepithelial voltage and resistance from which the equivalent short circuit current (I(sc)) was obtained. The apical and basolateral sides were independently perfused with adenosine and adenosine 5'-triphosphate (ATP). Adenosine (10(-5) M) had no effect on I(sc) at either the apical or basolateral side of vestibular dark cells and strial marginal cells, suggesting either the absence of P(1) receptors or the absence of coupling of P(1) receptors to vectorial ion transport by these epithelia. Apical perfusion of ATP (10(-8) to 10(-4) M) caused a decrease in I(sc) of both vestibular dark cells and strial marginal cells. Apical perfusion of the nucleotides uridine 5'-triphosphate (UTP), 2-methylthioadenosine triphosphate (2-meS-ATP), adenosine 5'-O-(3-thiotriphosphate) (ATPγS) and α,β-methylene adenosine 5'-triphosphate (α,β-meth-ATP) caused qualitatively similar responses with different magnitudes of response. The sequence of the magnitude of response of each compound at 10(-6) or 10(-5) M was assessed from the fractional change of I(sc). The sequence for vestibular dark cells was UTP = ATP = ATPγS ≫ 2-meS-ATP > α,β-meth-ATP, and for strial marginal cells it was UTP = ATP ≫ 2-meS-ATP, corresponding to the sequence for the P(2U) receptor. The effect of agonist on the apical membrane was reduced by the antagonist 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid (DIDS) but not cibacron blue or suramin. DIDS in the absence of exogenous purinergic agonist caused a sustained increase in I(sc). The effect of ATP on the apical membrane was greater in the absence of divalent cations. Basolateral perfusion of ATP led to a biphasic response of I(sc) in vestibular dark cell and strial marginal cell epithelia, consisting of an initial rapid increase followed by a slower decrease. Perfusion of the perilymphatic surface of the stria vascularis (basal cell layer) with ATP had no acute effect on I(sc). The initial increase of I(sc) in vestibular dark cell epithelium during basolateral perfusion had a sequence of 2-meS-ATP > ATP ≫ UTP = α,β-meth-ATP = ATPγS, corresponding to the sequence for the P(2Y) receptor. Subsequently, the agonists caused a sustained decrease in I(sc) with a sequence of ATPγS > 2-meS-ATP > ATP > UTP >α,β-meth-ATP. This sequence is most simply interpreted as the result of the coexistence of P(2U) and P(2Y) receptors in the basolateral membrane. Both the increase and decrease of I(sc) by ATP at the basolateral membrane were reduced by the antagonist suramin. These findings provide evidence for the regulation of transepithelial ion transport by P(2U) receptors in the apical membrane and by coexisting P(2U) and P(2Y) receptors in the basolateral membrane of K(+)-secretory epithelial cells in the inner ear and are consistent with the hypothesis that the apical receptors are part of an autocrine negative feedback system in these cells.
Figures

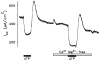
References
-
- Aubert A, Norris CH, Guth PS. Influence of ATP and ATP agonists on the physiology of the isolated semicircular canal of the frog (Rana pipiens) Neuroscience. 1994;62:963–974. - PubMed
-
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. - PubMed
-
- Boyer JL, Lazarowski ER, Chen XH, Harden TK. Identification of a P2Y-purinergic receptor that inhibits adenylyl cyclase. J Pharmacol Exp Ther. 1993;267:1140–1146. - PubMed
-
- Bruner G, Murphy S. ATP-evoked arachidonic acid mobilization in astrocytes is via a P2Y-purinergic receptor. J Neurochem. 1990;55:1569–1575. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
