Alkalistable endo-β-1,4-xylanase production from a newly isolated alkalitolerant Penicillium sp. SS1 using agro-residues
- PMID: 22582149
- PMCID: PMC3339607
- DOI: 10.1007/s13205-011-0009-5
Alkalistable endo-β-1,4-xylanase production from a newly isolated alkalitolerant Penicillium sp. SS1 using agro-residues
Abstract
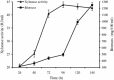
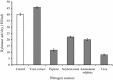
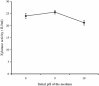
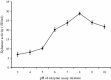
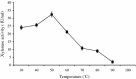
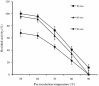
Thermostable and alkalitolerant xylanases have got intense research focus due to their vast applications in various industries including pulp and paper, food, feed, textile, biofuel, etc. In the present investigation, a Penicillum sp. SS1 isolated from degrading woody material was found to produce moderately thermoactive and alkalistable endo-β-1,4-xylanase (xylanase). Maximum xylanase production was observed after fourth day of fermentation (43.84 IU/ml). The organism produced substantial quantities of xylanase using agricultural residues like wheat bran (20.6 IU/ml), rice bran (21.8 IU/ml) and sawdust (10.7 IU/ml) as carbon sources. The enzyme preparation was totally free of filter paper activity (FPase) and possessed negligible carboxymethyl cellulase (CMCase) activity; this could be an important feature of enzyme if the intended application of enzyme is in pulp and paper industries. Among nitrogen sources examined, yeast extract supported maximum xylanase production (45.74 IU/ml), and was followed by soybean meal (22.2 IU/ml) and ammonium sulphate (20 IU/ml). Maximum xylanase production was observed at initial medium pH 9 (25.6 IU/ml); however, at pH 8 and 10 also significantly high enzyme titre was observed (24 and 21.2 IU/ml, respectively). Thus, Penicillium sp. SS1 displayed capability of growing and producing xylanase at high alkaline pH (8-10). Maximum xylanase activity was reported at 50 °C, however, significantly high activity was observed at 60 °C (65.4%), however, at 70-80 °C activity was lost considerably. At 50-60 °C the enzyme retained very high activity up to 30-60 min (91-100%), however, prolonged incubation (90 min) caused considerable activity reduction (residual activity 63-68%).
Figures

References
-
- Bakir U, Yavascoglu S, Guvence F, Erroyin A. An endo-β-1, 4-xylanase from Rhizopus oryzae: production, partial purification and biochemical characterization. Enzym Microb Technol. 2001;29:328–334. doi: 10.1016/S0141-0229(01)00379-9. - DOI
LinkOut - more resources
Full Text Sources

