Characterization of the [2Fe-2S] cluster of Escherichia coli transcription factor IscR
- PMID: 22583201
- PMCID: PMC3447993
- DOI: 10.1021/bi3003204
Characterization of the [2Fe-2S] cluster of Escherichia coli transcription factor IscR
Abstract
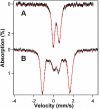
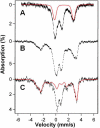
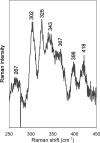
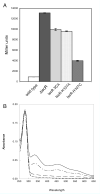
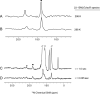
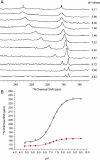
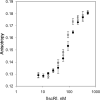
IscR is an Fe-S cluster-containing transcription factor involved in a homeostatic mechanism that controls Fe-S cluster biogenesis in Escherichia coli. Although IscR has been proposed to act as a sensor of the cellular demands for Fe-S cluster biogenesis, the mechanism by which IscR performs this function is not known. In this study, we investigated the biochemical properties of the Fe-S cluster of IscR to gain insight into the proposed sensing activity. Mössbauer studies revealed that IscR contains predominantly a reduced [2Fe-2S](+) cluster in vivo. However, upon anaerobic isolation of IscR, some clusters became oxidized to the [2Fe-2S](2+) form. Cluster oxidation did not, however, alter the affinity of IscR for its binding site within the iscR promoter in vitro, indicating that the cluster oxidation state is not important for regulation of DNA binding. Furthermore, characterization of anaerobically isolated IscR using resonance Raman, Mössbauer, and nuclear magnetic resonance spectroscopies leads to the proposal that the [2Fe-2S] cluster does not have full cysteinyl ligation. Mutagenesis studies indicate that, in addition to the three previously identified cysteine residues (Cys92, Cys98, and Cys104), the highly conserved His107 residue is essential for cluster ligation. Thus, these data suggest that IscR binds the cluster with an atypical ligation scheme of three cysteines and one histidine, a feature that may be relevant to the proposed function of IscR as a sensor of cellular Fe-S cluster status.
Figures

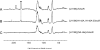
References
-
- Giel JL, Rodionov D, Liu MZ, Blattner FR, Kiley PJ. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol. Microbiol. 2006;60:1058–1075. - PubMed
-
- Yeo WS, Lee JH, Lee KC, Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol. Microbiol. 2006;61:206–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

