Vascular-targeted photothermal therapy of an orthotopic murine glioma model
- PMID: 22583571
- PMCID: PMC5375028
- DOI: 10.2217/nnm.11.189
Vascular-targeted photothermal therapy of an orthotopic murine glioma model
Abstract
Aim: To develop nanoshells for vascular-targeted photothermal therapy of glioma.
Materials & methods: The ability of nanoshells conjugated to VEGF and/or poly(ethylene glycol) (PEG) to thermally ablate VEGF receptor-2-positive endothelial cells upon near-infrared laser irradiation was evaluated in vitro. Subsequent in vivo studies evaluated therapy in mice bearing intracerebral glioma tumors by exposing tumors to near-infrared light after systemically delivering saline, PEG-coated nanoshells, or VEGF-coated nanoshells. The treatment effect was monitored with intravital microscopy and histology.
Results: VEGF-coated but not PEG-coated nanoshells bound VEGF receptor-2-positive cells in vitro to enable targeted photothermal ablation. In vivo, VEGF targeting doubled the proportion of nanoshells bound to tumor vessels and vasculature was disrupted following laser exposure. Vessels were not disrupted in mice that received saline. The normal brain was unharmed in all treatment and control mice.
Conclusion: Nanoshell therapy can induce vascular disruption in glioma.
Figures
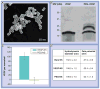

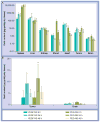




Similar articles
-
Nanoshell-mediated photothermal therapy improves survival in a murine glioma model.J Neurooncol. 2011 Aug;104(1):55-63. doi: 10.1007/s11060-010-0470-8. Epub 2010 Nov 26. J Neurooncol. 2011. PMID: 21110217 Free PMC article.
-
Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines.J Neurooncol. 2008 Jan;86(2):165-72. doi: 10.1007/s11060-007-9467-3. Epub 2007 Sep 6. J Neurooncol. 2008. PMID: 17805488
-
Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells.J Neurooncol. 2011 Sep;104(2):439-48. doi: 10.1007/s11060-010-0511-3. Epub 2011 Jan 9. J Neurooncol. 2011. PMID: 21221712 Free PMC article.
-
Macrophages as cell-based delivery systems for nanoshells in photothermal therapy.Ann Biomed Eng. 2012 Feb;40(2):507-15. doi: 10.1007/s10439-011-0415-1. Epub 2011 Oct 7. Ann Biomed Eng. 2012. PMID: 21979168 Free PMC article. Review.
-
Angiogenesis in malignant gliomas.Glia. 1995 Nov;15(3):339-47. doi: 10.1002/glia.440150313. Glia. 1995. PMID: 8586468 Review.
Cited by
-
Carbon-Coated Magnetic Nanoparticle Dedicated to MRI/Photoacoustic Imaging of Tumor in Living Mice.Front Bioeng Biotechnol. 2021 Dec 2;9:800744. doi: 10.3389/fbioe.2021.800744. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34926438 Free PMC article.
-
Peptides and proteins used to enhance gold nanoparticle delivery to the brain: preclinical approaches.Int J Nanomedicine. 2015 Aug 10;10:4919-36. doi: 10.2147/IJN.S82310. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26300639 Free PMC article. Review.
-
Photothermal Therapy for the Treatment of Glioblastoma: Potential and Preclinical Challenges.Front Oncol. 2021 Jan 15;10:610356. doi: 10.3389/fonc.2020.610356. eCollection 2020. Front Oncol. 2021. PMID: 33520720 Free PMC article. Review.
-
Research Progress on Gene Editing Based on Nano-Drug Delivery Vectors for Tumor Therapy.Front Bioeng Biotechnol. 2022 Mar 28;10:873369. doi: 10.3389/fbioe.2022.873369. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35419357 Free PMC article. Review.
-
Antitumor therapeutic application of self-assembled RNAi-AuNP nanoconstructs: Combination of VEGF-RNAi and photothermal ablation.Theranostics. 2017 Jan 1;7(1):9-22. doi: 10.7150/thno.16042. eCollection 2017. Theranostics. 2017. PMID: 28042312 Free PMC article.
References
-
- Tuettenberg J, Friedel C, Vajkoczy P. Angiogenesis in malignant glioma – a target for antitumor therapy? Crit Rev Oncol Hematol. 2006;59(3):181–193. - PubMed
-
- Day E, Thompson P, Zhang L, et al. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2011;104(1):55–63. Our previous work demonstrated that treatment of mice bearing subcutaneous glioma tumors with nanoshell-mediated photothermal therapy can induce tumor regression and prolong survival. - PMC - PubMed
-
- Day ES, Morton JG, West JL. Nanoparticles for thermal cancer therapy. J Biomech Eng. 2009;131(7):074001–074005. - PubMed
-
- Kennedy LC, Bickford LR, Lewinski NA, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7(2):169–183. - PubMed
-
- Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
