Prostate-targeted biodegradable nanoparticles loaded with androgen receptor silencing constructs eradicate xenograft tumors in mice
- PMID: 22583574
- PMCID: PMC3448843
- DOI: 10.2217/nnm.12.14
Prostate-targeted biodegradable nanoparticles loaded with androgen receptor silencing constructs eradicate xenograft tumors in mice
Abstract
Background: Prostate cancer is the major cause of cancer death in men and the androgen receptor (AR) has been shown to play a critical role in the progression of the disease. Our previous reports showed that knocking down the expression of the AR gene using a siRNA-based approach in prostate cancer cells led to apoptotic cell death and xenograft tumor eradication. In this study, we utilized a biodegradable nanoparticle to deliver the therapeutic AR shRNA construct specifically to prostate cancer cells.
Materials & methods: The biodegradable nanoparticles were fabricated using a poly(dl-lactic-co-glycolic acid) polymer and the AR shRNA constructs were loaded inside the particles. The surface of the nanoparticles were then conjugated with prostate-specific membrane antigen aptamer A10 for prostate cancer cell-specific targeting.
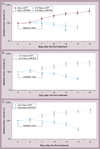
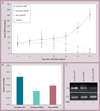
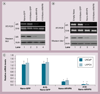
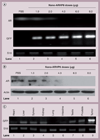
Results: A10-conjugation largely enhanced cellular uptake of nanoparticles in both cell culture- and xenograft-based models. The efficacy of AR shRNA encapsulated in nanoparticles on AR gene silencing was confirmed in PC-3/AR-derived xenografts in nude mice. The therapeutic property of A10-conjugated AR shRNA-loaded nanoparticles was evaluated in xenograft models with different prostate cancer cell lines: 22RV1, LAPC-4 and LNCaP. Upon two injections of the AR shRNA-loaded nanoparticles, rapid tumor regression was observed over 2 weeks. Consistent with previous reports, A10 aptamer conjugation significantly enhanced xenograft tumor regression compared with nonconjugated nanoparticles.
Discussion: These data demonstrated that tissue-specific delivery of AR shRNA using a biodegradable nanoparticle approach represents a novel therapy for life-threatening prostate cancers.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures



References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60(5):277–300. - PubMed
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005;23:8253–8261. - PubMed
-
- Li B, Thrasher JB. Androgen receptor and cellular survival in prostate cancer. Rec. Res. Dev. Cancer. 2005;7:65–89.
-
-
Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004;10(1):33–39. ▪▪ Comprehensive study showing the critical role of androgen receptor (AR) in prostate cancer progression.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
