The molecular basis of HIV entry
- PMID: 22583677
- PMCID: PMC3417324
- DOI: 10.1111/j.1462-5822.2012.01812.x
The molecular basis of HIV entry
Abstract
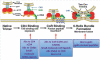
Infection by HIV starts when the virus attaches to a susceptible cell. For viral replication to continue, the viral envelope must fuse with a cellular membrane, thereby delivering the viral core to the cytoplasm, where the RNA genome is reverse-transcribed. The key players in this entry by fusion are the envelope glycoprotein, on the viral side, and CD4 and a co-receptor, CCR5 or CXCR4, on the cellular side. Here, the interplay of these molecules is reviewed from cell-biological, structural, mechanistic, and modelling-based perspectives. Hypotheses are evaluated regarding the cellular compartment for entry, the transfer of virus through direct cell-to-cell contact, the sequence of molecular events, and the number of molecules involved on each side of the virus-cell divide. An emerging theme is the heterogeneity among the entry mediators on both sides, a diversity that affects the efficacy of entry inhibitors, be they small-molecule ligands, peptides or neutralizing antibodies. These insights inform rational strategies for therapy as well as vaccination.
© 2012 Blackwell Publishing Ltd.
Figures





References
-
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

