Neuregulin-1 is neuroprotective in a rat model of organophosphate-induced delayed neuronal injury
- PMID: 22583949
- PMCID: PMC3607352
- DOI: 10.1016/j.taap.2012.05.001
Neuregulin-1 is neuroprotective in a rat model of organophosphate-induced delayed neuronal injury
Abstract
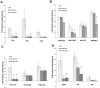
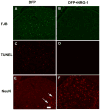
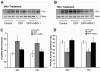
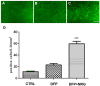
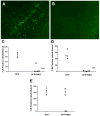
Current medical countermeasures against organophosphate (OP) nerve agents are effective in reducing mortality, but do not sufficiently protect the CNS from delayed brain damage and persistent neurological symptoms. In this study, we examined the efficacy of neuregulin-1 (NRG-1) in protecting against delayed neuronal cell death following acute intoxication with the OP diisopropylflurophosphate (DFP). Adult male Sprague-Dawley rats were pretreated with pyridostigmine (0.1 mg/kg BW, i.m.) and atropine methylnitrate (20 mg/kg BW, i.m.) prior to DFP (9 mg/kg BW, i.p.) intoxication to increase survival and reduce peripheral signs of cholinergic toxicity but not prevent DFP-induced seizures or delayed neuronal injury. Pretreatment with NRG-1 did not protect against seizures in rats exposed to DFP. However, neuronal injury was significantly reduced in most brain regions by pretreatment with NRG-1 isoforms NRG-EGF (3.2 μg/kg BW, i.a) or NRG-GGF2 (48 μg/kg BW, i.a.) as determined by FluroJade-B labeling in multiple brain regions at 24 h post-DFP injection. NRG-1 also blocked apoptosis and oxidative stress-mediated protein damage in the brains of DFP-intoxicated rats. Administration of NRG-1 at 1h after DFP injection similarly provided significant neuroprotection against delayed neuronal injury. These findings identify NRG-1 as a promising adjuvant therapy to current medical countermeasures for enhancing neuroprotection against acute OP intoxication.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

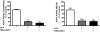


References
-
- Carlsson T, Schindler FR, Hollerhage M, Depboylu C, Arias-Carrion O, Schnurrbusch S, Rosler TW, Wozny W, Schwall GP, Groebe K, Oertel WH, Brundin P, Schrattenholz A, Hoglinger GU. Systemic administration of neuregulin-1beta(1) protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neurochem. 2011;117:1066–1074. - PubMed
-
- Collombet JM, Masqueliez C, Four E, Burckhart MF, Bernabe D, Baubichon D, Lallement G. Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci Lett. 2006;398:337–342. - PubMed
-
- De Sarro G, Di Paola ED, De Sarro A, Vidal MJ. L-arginine potentiates excitatory amino acid-induced seizures elicited in the deep prepiriform cortex. Eur J Pharmacol. 1993;230:151–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 NS034194/NS/NINDS NIH HHS/United States
- G12 RR003034/RR/NCRR NIH HHS/United States
- R21 NS072094/NS/NINDS NIH HHS/United States
- U01 NS057993/NS/NINDS NIH HHS/United States
- U54 NS046798/NS/NINDS NIH HHS/United States
- G12 RR03034/RR/NCRR NIH HHS/United States
- G20 RR014335/RR/NCRR NIH HHS/United States
- G20 RR14335/RR/NCRR NIH HHS/United States
- S21 MD000101/MD/NIMHD NIH HHS/United States
- U54 NS060659/NS/NINDS NIH HHS/United States
- C06 RR07571/RR/NCRR NIH HHS/United States
- U54 NS079202/NS/NINDS NIH HHS/United States

