GQ-16, a novel peroxisome proliferator-activated receptor γ (PPARγ) ligand, promotes insulin sensitization without weight gain
- PMID: 22584573
- PMCID: PMC3431672
- DOI: 10.1074/jbc.M111.332106
GQ-16, a novel peroxisome proliferator-activated receptor γ (PPARγ) ligand, promotes insulin sensitization without weight gain
Abstract
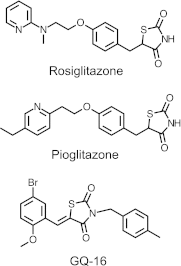
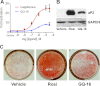
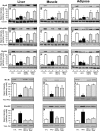
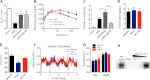
The recent discovery that peroxisome proliferator-activated receptor γ (PPARγ) targeted anti-diabetic drugs function by inhibiting Cdk5-mediated phosphorylation of the receptor has provided a new viewpoint to evaluate and perhaps develop improved insulin-sensitizing agents. Herein we report the development of a novel thiazolidinedione that retains similar anti-diabetic efficacy as rosiglitazone in mice yet does not elicit weight gain or edema, common side effects associated with full PPARγ activation. Further characterization of this compound shows GQ-16 to be an effective inhibitor of Cdk5-mediated phosphorylation of PPARγ. The structure of GQ-16 bound to PPARγ demonstrates that the compound utilizes a binding mode distinct from other reported PPARγ ligands, although it does share some structural features with other partial agonists, such as MRL-24 and PA-082, that have similarly been reported to dissociate insulin sensitization from weight gain. Hydrogen/deuterium exchange studies reveal that GQ-16 strongly stabilizes the β-sheet region of the receptor, presumably explaining the compound's efficacy in inhibiting Cdk5-mediated phosphorylation of Ser-273. Molecular dynamics simulations suggest that the partial agonist activity of GQ-16 results from the compound's weak ability to stabilize helix 12 in its active conformation. Our results suggest that the emerging model, whereby "ideal" PPARγ-based therapeutics stabilize the β-sheet/Ser-273 region and inhibit Cdk5-mediated phosphorylation while minimally invoking adipogenesis and classical agonism, is indeed a valid framework to develop improved PPARγ modulators that retain antidiabetic actions while minimizing untoward effects.
Figures



References
-
- Lehmann J. M., Moore L. B., Smith-Oliver T. A., Wilkison W. O., Willson T. M., Kliewer S. A. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J. Biol. Chem. 270, 12953–12956 - PubMed
-
- Nissen S. E., Wolski K. (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 - PubMed
-
- Grey A., Bolland M., Gamble G., Wattie D., Horne A., Davidson J., Reid I. R. (2007) The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women. A randomized, controlled trial. J. Clin. Endocrinol. Metab. 92, 1305–1310 - PubMed
-
- Morrison R. F., Farmer S. R. (2000) Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 130, 3116S–3121S - PubMed
-
- Tontonoz P., Hu E., Spiegelman B. M. (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147–1156 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

