Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses
- PMID: 22584577
- PMCID: PMC3408134
- DOI: 10.1074/jbc.M111.330902
Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses
Abstract
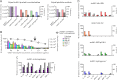
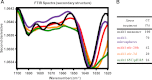
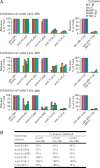
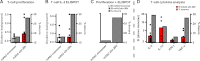
Aggregation of biotherapeutics has the potential to induce an immunogenic response. Here, we show that aggregated therapeutic antibodies, previously generated and determined to contain a variety of attributes (Joubert, M. K., Luo, Q., Nashed-Samuel, Y., Wypych, J., and Narhi, L. O. (2011) J. Biol. Chem. 286, 25118-25133), can enhance the in vitro innate immune response of a population of naive human peripheral blood mononuclear cells. This response depended on the aggregate type, inherent immunogenicity of the monomer, and donor responsiveness, and required a high number of particles, well above that detected in marketed drug products, at least in this in vitro system. We propose a cytokine signature as a potential biomarker of the in vitro peripheral blood mononuclear cell response to aggregates. The cytokines include IL-1β, IL-6, IL-10, MCP-1, MIP-1α, MIP-1β, MMP-2, and TNF-α. IL-6 and IL-10 might have an immunosuppressive effect on the long term immune response. Aggregates made by stirring induced the highest response compared with aggregates made by other methods. Particle size in the 2-10 μm range and the retention of some folded structure were associated with an increased response. The mechanism of aggregate activation at the innate phase was found to occur through specific cell surface receptors (the toll-like receptors TLR-2 and TLR-4, FcγRs, and the complement system). The innate signal was shown to progress to an adaptive T-cell response characterized by T-cell proliferation and secretion of T-cell cytokines. Investigating the ability of aggregates to induce cytokine signatures as biomarkers of immune responses is essential for determining their risk of immunogenicity.
Figures


References
-
- Carpenter J. F., Randolph T. W., Jiskoot W., Crommelin D. J., Middaugh C. R., Winter G., Fan Y. X., Kirshner S., Verthelyi D., Kozlowski S., Clouse K. A., Swann P. G., Rosenberg A., Cherney B. (2009) Overlooking subvisible particles in therapeutic protein products. Gaps that may compromise product quality. J. Pharm. Sci. 98, 1201–1205 - PMC - PubMed
-
- Singh S. K. (2011) Impact of product-related factors on immunogenicity of biotherapeutics. J. Pharm. Sci. 100, 354–387 - PubMed
-
- Demeule B., Gurny R., Arvinte T. (2006) Where disease pathogenesis meets protein formulation. Renal deposition of immunoglobulin aggregates. Eur. J. Pharm. Biopharm. 62, 121–130 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

