Quantification, self-renewal, and genetic tracing of FL1⁺ tumor-initiating cells in a large cohort of human gliomas
- PMID: 22584872
- PMCID: PMC3367853
- DOI: 10.1093/neuonc/nos084
Quantification, self-renewal, and genetic tracing of FL1⁺ tumor-initiating cells in a large cohort of human gliomas
Retraction in
-
Retraction: Quantification, self-renewal, and genetic tracing of FL1⁺ tumor-initiating cells in a large cohort of human gliomas. Neuro-Oncology 14(6):720-35, 2012.Neuro Oncol. 2014 Jun;16(6):889. doi: 10.1093/neuonc/nou086. Neuro Oncol. 2014. PMID: 24832621 Free PMC article. No abstract available.
Abstract
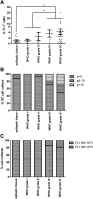
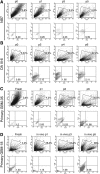
Evidence has emerged that the initiation and growth of gliomas is sustained by a subpopulation of cancer-initiating cells (CICs). Because of the difficulty of using markers to tag CICs in gliomas, we have previously exploited more robust phenotypic characteristics, including a specific morphology and intrincic autofluorescence, to identify and isolate a subpopulation of glioma CICs, called FL1(+). The objective of this study was to further validate our method in a large cohort of human glioma and a mouse model of glioma. Seventy-four human gliomas of all grades and the GFAP-V(12)HA-ras B8 mouse model were analyzed for in vitro self-renewal capacity and their content of FL1(+). Nonneoplastic brain tissue and embryonic mouse brain were used as control. Genetic traceability along passages was assessed with microsatellite analysis. We found that FL1(+) cells from low-grade gliomas and from control nonneoplasic brain tissue show a lower level of autofluorescence and undergo a restricted number of cell divisions before dying in culture. In contrast, we found that FL1(+) cells derived from many but not all high-grade gliomas acquire high levels of autofluorescence and can be propagated in long-term cultures. Moreover, FL1(+) cells show a remarkable traceability over time in vitro and in vivo. Our results show that FL1(+) cells can be found in all specimens of a large cohort of human gliomas of different grades and in a model of genetically induced mouse glioma as well as nonneoplastic brain. However, their self-renewal capacity is variable and seems to be dependent on the tumor grade.
Figures


Similar articles
-
Marker-independent identification of glioma-initiating cells.Nat Methods. 2010 Mar;7(3):224-8. doi: 10.1038/nmeth.1430. Epub 2010 Feb 21. Nat Methods. 2010. Retraction in: Nat Methods. 2013 Oct;10(10):1035. doi: 10.1038/nmeth1013-1035c. PMID: 20173750 Retracted.
-
Mouse induced glioma-initiating cell models and therapeutic targets.Anticancer Agents Med Chem. 2010 Jul;10(6):471-80. doi: 10.2174/1871520611009060471. Anticancer Agents Med Chem. 2010. PMID: 20879984
-
Isolation of glioma cancer stem cells in relation to histological grades in glioma specimens.Childs Nerv Syst. 2013 Feb;29(2):217-29. doi: 10.1007/s00381-012-1964-9. Epub 2012 Nov 10. Childs Nerv Syst. 2013. PMID: 23143002
-
The SVZ and Its Relationship to Stem Cell Based Neuro-oncogenesis.Adv Exp Med Biol. 2015;853:23-32. doi: 10.1007/978-3-319-16537-0_2. Adv Exp Med Biol. 2015. PMID: 25895705 Review.
-
Characteristics of glioma stem cells.Brain Tumor Pathol. 2013 Oct;30(4):209-14. doi: 10.1007/s10014-013-0141-5. Epub 2013 Apr 13. Brain Tumor Pathol. 2013. PMID: 23584571 Review.
References
-
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi:10.1158/0008-5472.CAN-04-1364. - DOI - PubMed
-
- Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–9400. doi:10.1038/sj.onc.1208311. - DOI - PubMed
-
- Kondo T. Brain cancer stem-like cells. Eur J Cancer. 2006;42(9):1237–1242. doi:10.1016/j.ejca.2006.01.038. - DOI - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi:10.1038/35102167. - DOI - PubMed
-
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi:10.1038/nrc1232. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

