The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes
- PMID: 22585215
- PMCID: PMC3393457
- DOI: 10.1128/AAC.00693-12
The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes
Abstract
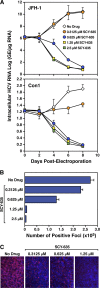
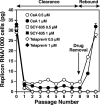
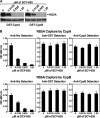
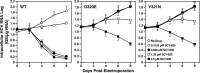
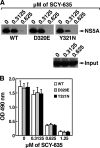
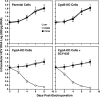
The nonimmunosuppressive cyclophilin (Cyp) inhibitor SCY-635 blocks hepatitis C virus (HCV) replication both in vitro and in vivo and represents a novel potent anti-HCV agent. However, its mechanism of action remains to be fully elucidated. A growing body of evidence suggests that cyclophilin A (CypA) is absolutely necessary for HCV replication and that the HCV nonstructural 5A (NS5A) protein serves as a main viral ligand for CypA. In this study, we examined the effect of SCY-635 on HCV replication. Specifically, we asked whether SCY-635 blocks HCV replication by targeting CypA-NS5A interactions. We also investigated the possibility that HCV can escape SCY-635 selection pressure and whether this resistance influences either CypA-NS5A interactions or the dependence of HCV on CypA. We found not only that SCY-635 efficiently inhibits HCV replication, but it is sufficient alone to clear HCV replicon-containing cells. We found that SCY-635 prevents CypA-NS5A interactions in a dose-dependent manner. SCY-635 prevents the contact between CypA and NS5A derived from genotypes 1 to 3. Together, these data suggest that NS5A-CypA interactions control HCV replication and that SCY-635 blocks viral replication by preventing the formation of these complexes. We also found that NS5A mutant proteins found in SCY-635-resistant HCV replicons behave similarly to wild-type NS5A in terms of both CypA binding and SCY-635-mediated dissociation and inhibition of CypA binding. However, the NS5A mutations found in SCY-635-resistant HCV replicons rescued viral replication in CypA-knockdown cells, suggesting that the NS5A mutations, which arose in vitro under SCY-635 selection, do not alter the binding affinity of CypA for NS5A. These specific mutations in NS5A eliminate the dependence of HCV RNA replication on the expression of host CypA.
Figures

Similar articles
-
HCV resistance to cyclosporin A does not correlate with a resistance of the NS5A-cyclophilin A interaction to cyclophilin inhibitors.J Hepatol. 2010 Jul;53(1):50-6. doi: 10.1016/j.jhep.2010.01.041. Epub 2010 Apr 3. J Hepatol. 2010. PMID: 20451281 Free PMC article.
-
Inhibition of HCV replication by cyclophilin antagonists is linked to replication fitness and occurs by inhibition of membranous web formation.Gastroenterology. 2014 May;146(5):1361-72.e1-9. doi: 10.1053/j.gastro.2014.01.055. Epub 2014 Jan 31. Gastroenterology. 2014. PMID: 24486951
-
ISGylation of Hepatitis C Virus NS5A Protein Promotes Viral RNA Replication via Recruitment of Cyclophilin A.J Virol. 2020 Sep 29;94(20):e00532-20. doi: 10.1128/JVI.00532-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32727878 Free PMC article.
-
Cyclophilin inhibitors for the treatment of HCV infection.Curr Opin Investig Drugs. 2010 Aug;11(8):911-8. Curr Opin Investig Drugs. 2010. PMID: 20721833 Review.
-
Cyclophilin A as a New Therapeutic Target for Hepatitis C Virus-induced Hepatocellular Carcinoma.Korean J Physiol Pharmacol. 2013 Oct;17(5):375-83. doi: 10.4196/kjpp.2013.17.5.375. Epub 2013 Oct 17. Korean J Physiol Pharmacol. 2013. PMID: 24227937 Free PMC article. Review.
Cited by
-
HCV NS5A dimer interface residues regulate HCV replication by controlling its self-interaction, hyperphosphorylation, subcellular localization and interaction with cyclophilin A.PLoS Pathog. 2018 Jul 23;14(7):e1007177. doi: 10.1371/journal.ppat.1007177. eCollection 2018 Jul. PLoS Pathog. 2018. PMID: 30036383 Free PMC article.
-
Cyclophilin Inhibitors Remodel the Endoplasmic Reticulum of HCV-Infected Cells in a Unique Pattern Rendering Cells Impervious to a Reinfection.PLoS One. 2016 Jul 21;11(7):e0159511. doi: 10.1371/journal.pone.0159511. eCollection 2016. PLoS One. 2016. PMID: 27442520 Free PMC article.
-
Current and future therapies for hepatitis C virus infection.N Engl J Med. 2013 May 16;368(20):1907-17. doi: 10.1056/NEJMra1213651. N Engl J Med. 2013. PMID: 23675659 Free PMC article. Review.
-
Cyclophilin inhibition as a strategy for the treatment of human disease.Front Pharmacol. 2024 Jul 8;15:1417945. doi: 10.3389/fphar.2024.1417945. eCollection 2024. Front Pharmacol. 2024. PMID: 39045055 Free PMC article. Review.
-
Hepatitis C NS5A protein: two drug targets within the same protein with different mechanisms of resistance.Curr Opin Virol. 2014 Oct;8:30-7. doi: 10.1016/j.coviro.2014.04.012. Epub 2014 May 27. Curr Opin Virol. 2014. PMID: 24879295 Free PMC article. Review.
References
-
- Armstrong GL, et al. 2006. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144:705–714 - PubMed
-
- Asselah T, Benhamou Y, Marcellin P. 2009. Protease and polymerase inhibitors for the treatment of hepatitis C. Liver Int. 29(Suppl 1):57–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

