Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model
- PMID: 22585229
- PMCID: PMC3421617
- DOI: 10.1128/AAC.00360-12
Amixicile, a novel inhibitor of pyruvate: ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model
Abstract
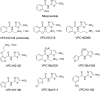
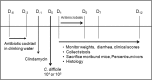
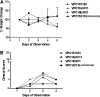
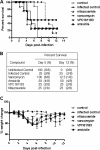
Clostridium difficile infection (CDI) is a serious diarrheal disease that often develops following prior antibiotic usage. One of the major problems with current therapies (oral vancomycin and metronidazole) is the high rate of recurrence. Nitazoxanide (NTZ), an inhibitor of pyruvate:ferredoxin oxidoreductase (PFOR) in anaerobic bacteria, parasites, Helicobacter pylori, and Campylobacter jejuni, also shows clinical efficacy against CDI. From a library of ∼250 analogues of NTZ, we identified leads with increased potency for PFOR. MIC screens indicated in vitro activity in the 0.05- to 2-μg/ml range against C. difficile. To improve solubility, we replaced the 2-acetoxy group with propylamine, producing amixicile, a soluble (10 mg/ml), nontoxic (cell-based assay) lead that produced no adverse effects in mice by oral or intraperitoneal (i.p.) routes at 200 mg/kg of body weight/day. In initial efficacy testing in mice treated (20 mg/kg/day, 5 days each) 1 day after receiving a lethal inoculum of C. difficile, amixicile showed slightly less protection than did vancomycin by day 5. However, in an optimized CDI model, amixicile showed equivalence to vancomycin and fidaxomicin at day 5 and there was significantly greater survival produced by amixicile than by the other drugs on day 12. All three drugs were comparable by measures of weight loss/gain and severity of disease. Recurrence of CDI was common for mice treated with vancomycin or fidaxomicin but not for mice receiving amixicile or NTZ. These results suggest that gut repopulation with beneficial (non-PFOR) bacteria, considered essential for protection against CDI, rebounds much sooner with amixicile therapy than with vancomycin or fidaxomicin. If the mouse model is indeed predictive of human CDI disease, then amixicile, a novel PFOR inhibitor, appears to be a very promising new candidate for treatment of CDI.
Figures

Similar articles
-
Synthesis and Antimicrobial Evaluation of Amixicile-Based Inhibitors of the Pyruvate-Ferredoxin Oxidoreductases of Anaerobic Bacteria and Epsilonproteobacteria.Antimicrob Agents Chemother. 2016 Jun 20;60(7):3980-7. doi: 10.1128/AAC.00670-16. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27090174 Free PMC article.
-
Preclinical studies of amixicile, a systemic therapeutic developed for treatment of Clostridium difficile infections that also shows efficacy against Helicobacter pylori.Antimicrob Agents Chemother. 2014 Aug;58(8):4703-12. doi: 10.1128/AAC.03112-14. Epub 2014 Jun 2. Antimicrob Agents Chemother. 2014. PMID: 24890599 Free PMC article.
-
Fidaxomicin versus metronidazole, vancomycin and their combination for initial episode, first recurrence and severe Clostridioides difficile infection - An observational cohort study.Int J Infect Dis. 2021 Feb;103:226-233. doi: 10.1016/j.ijid.2020.11.004. Epub 2020 Nov 11. Int J Infect Dis. 2021. PMID: 33188906
-
Clostridium difficile infection: update on emerging antibiotic treatment options and antibiotic resistance.Expert Rev Anti Infect Ther. 2010 May;8(5):555-64. doi: 10.1586/eri.10.28. Expert Rev Anti Infect Ther. 2010. PMID: 20455684 Free PMC article. Review.
-
Fidaxomicin: a novel macrocyclic antibiotic for the treatment of Clostridium difficile infection.Am J Health Syst Pharm. 2012 Jun 1;69(11):933-43. doi: 10.2146/ajhp110371. Am J Health Syst Pharm. 2012. PMID: 22610025 Review.
Cited by
-
New functions of pirin proteins and a 2-ketoglutarate: Ferredoxin oxidoreductase ortholog in Bacteroides fragilis metabolism and their impact on antimicrobial susceptibility to metronidazole and amixicile.Microbiologyopen. 2024 Aug;13(4):e1429. doi: 10.1002/mbo3.1429. Microbiologyopen. 2024. PMID: 39109824 Free PMC article.
-
Synthesis and Antimicrobial Evaluation of Amixicile-Based Inhibitors of the Pyruvate-Ferredoxin Oxidoreductases of Anaerobic Bacteria and Epsilonproteobacteria.Antimicrob Agents Chemother. 2016 Jun 20;60(7):3980-7. doi: 10.1128/AAC.00670-16. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27090174 Free PMC article.
-
Multimodal vaccination targeting the receptor binding domains of Clostridioides difficile toxins A and B with an attenuated Salmonella Typhimurium vector (YS1646) protects mice from lethal challenge.Microbiol Spectr. 2024 Feb 6;12(2):e0310922. doi: 10.1128/spectrum.03109-22. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189293 Free PMC article.
-
Role of interleukin 23 signaling in Clostridium difficile colitis.J Infect Dis. 2013 Sep;208(6):917-20. doi: 10.1093/infdis/jit277. Epub 2013 Jun 17. J Infect Dis. 2013. PMID: 23776194 Free PMC article.
-
Intestinal Inflammation Reversibly Alters the Microbiota to Drive Susceptibility to Clostridioides difficile Colonization in a Mouse Model of Colitis.mBio. 2022 Aug 30;13(4):e0190422. doi: 10.1128/mbio.01904-22. Epub 2022 Jul 28. mBio. 2022. PMID: 35900107 Free PMC article.
References
-
- Carroll KC, Bartlett JG. 2011. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu. Rev. Microbiol. 65:501–521 - PubMed
-
- Chabrière E, Volbeda A, Fontecilla-Camps JC, Roth M, Charon MH. 1999. Combination of methods used in the structure solution of pyruvate:ferredoxin oxidoreductase from two crystal forms. Acta Crystallogr. D Biol. Crystallogr. 55:1546–1554 - PubMed
-
- Chen X, et al. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

