Determination of nicotine absorption from multiple tobacco products and nicotine gum
- PMID: 22585541
- PMCID: PMC3524070
- DOI: 10.1093/ntr/nts123
Determination of nicotine absorption from multiple tobacco products and nicotine gum
Abstract
Introduction: Snus is a smokeless tobacco product traditionally used in Scandinavia and available in pouched or loose forms. The objective of this study was to determine nicotine absorption for current pouched and loose snus products in comparison with a cigarette and an over-the-counter nicotine gum.
Methods: We conducted an open-label, randomized, 6-way, crossover study involving 20 healthy snus and cigarette users. One of 6 products (2 pouched snus, 2 weights of loose snus, a cigarette, and a nicotine gum) was administered at each of 6 visits. Blood samples were taken at intervals over 120 min and sensory perception assessed by questionnaire.
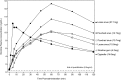
Results: For the 4 smokeless tobacco products and the nicotine gum, blood plasma levels of nicotine were ranked according to total nicotine content as follows: loose snus (27.1 mg nicotine) > pouched snus (14.7 mg nicotine) > loose snus (10.8 mg nicotine) = pouched snus (10.7 mg nicotine) > nicotine gum (4.2 mg nicotine). The area under the plasma concentration-time curve (AUC) and maximum plasma concentration (C(max)) of nicotine ranged from 26.9 to 13.1 ng.h/ml and 17.9 to 9.1 ng.h/ml, respectively across all the products. Nicotine was absorbed more rapidly from the cigarette but systemic exposure was within the range of the smokeless tobacco products (AUC = 14.8 ng.h/ml; C(max) = 12.8 ng.h/ml).
Conclusions: This study has generated new information on comparative nicotine absorption from a cigarette, loose snus, and pouched snus typical of products sold in Scandinavia. The similar nicotine absorption for 1 g portions of loose and pouched snus with approximately 11 mg of nicotine indicate that absorption kinetics were dependent on quantity of tobacco by weight and total nicotine content rather than product form.
Figures
References
-
- Andersson G, Bjornberg G, Curvall M. Oral mucosal changes and nicotine disposition in users of Swedish smokeless tobacco products: A comparative study. Journal of Oral Pathology & Medicine. 1994;23:161–167. doi:org/10.1111/j.1600-0714.1994.tb01106.x. - PubMed
-
- Benowitz NL, Swan GE, Jacob P, III, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clinical Pharmacology & Therapeutics. 2006;80:457–467. doi:10.1016/j.clpt.2006.08.011. - PubMed
-
- Benowitz NL, Porchet H, Sheiner L, Jacob P. Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology & Therapeutics. 1988;44:23–28. doi:org/10.1038/clpt.1988.107. - PubMed
-
- Britton J. Should doctors advocate snus and other nicotine replacements? Yes. British Medical Journal. 2008;336:358. doi:org/10.1136/bmj.39479.427477.AD. - PMC - PubMed
-
- Committee for Medicinal Products for Human Use. Guideline on the investigation of bioequivalence. European medicines agency. 2010. Retrieved from http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideli....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

