Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection
- PMID: 22585691
- PMCID: PMC4517071
- DOI: 10.1200/JCO.2011.36.7136
Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection
Abstract
Purpose: Surgical resection of gastric cancer has produced suboptimal survival despite multiple randomized trials that used postoperative chemotherapy or more aggressive surgical procedures. We performed a randomized phase III trial of postoperative radiochemotherapy in those at moderate risk of locoregional failure (LRF) following surgery. We originally reported results with 4-year median follow-up. This update, with a more than 10-year median follow-up, presents data on failure patterns and second malignancies and explores selected subset analyses.
Patients and methods: In all, 559 patients with primaries ≥ T3 and/or node-positive gastric cancer were randomly assigned to observation versus radiochemotherapy after R0 resection. Fluorouracil and leucovorin were administered before, during, and after radiotherapy. Radiotherapy was given to all LRF sites to a dose of 45 Gy.
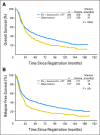
Results: Overall survival (OS) and relapse-free survival (RFS) data demonstrate continued strong benefit from postoperative radiochemotherapy. The hazard ratio (HR) for OS is 1.32 (95% CI, 1.10 to 1.60; P = .0046). The HR for RFS is 1.51 (95% CI, 1.25 to 1.83; P < .001). Adjuvant radiochemotherapy produced substantial reduction in both overall relapse and locoregional relapse. Second malignancies were observed in 21 patients with radiotherapy versus eight with observation (P = .21). Subset analyses show robust treatment benefit in most subsets, with the exception of patients with diffuse histology who exhibited minimal nonsignificant treatment effect.
Conclusion: Intergroup 0116 (INT-0116) demonstrates strong persistent benefit from adjuvant radiochemotherapy. Toxicities, including second malignancies, appear acceptable, given the magnitude of RFS and OS improvement. LRF reduction may account for the majority of overall relapse reduction. Adjuvant radiochemotherapy remains a rational standard therapy for curatively resected gastric cancer with primaries T3 or greater and/or positive nodes.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Adjuvant therapy for gastric cancer: revisiting the past to clarify the future.J Clin Oncol. 2012 Jul 1;30(19):2297-9. doi: 10.1200/JCO.2012.42.4069. Epub 2012 May 14. J Clin Oncol. 2012. PMID: 22585690 No abstract available.
-
[Despite of the updated analysis of the SWOG-directed Intergroup Study 0116, adjuvant radiochemotherapy is not yet the standard for gastric cancer after curative resection].Strahlenther Onkol. 2012 Nov;188(11):1052-3. doi: 10.1007/s00066-012-0218-9. Strahlenther Onkol. 2012. PMID: 23053155 German. No abstract available.
References
-
- Hundahl SA, Menck HR, Mansour EG, et al. The National Cancer Data Base report on gastric carcinoma. Cancer. 1997;80:2333–2341. - PubMed
-
- Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. - PubMed
-
- Dockerty M. Pathologic aspects of primary malignant neoplasms of the stomach. In: ReMine W, Priestley J, Berkson J, editors. Cancer of the Stomach. Philadelphia, PA: WB Saunders; 1964. p. 173.
-
- Gunderson LL, Sosin H. Adenocarcinoma of the stomach: Areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- U10 CA045461/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- U10 CA016385/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- U10 CA063845/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA045450/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- U10 CA035262/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA037422/CA/NCI NIH HHS/United States
- U10 CA058686/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- U10 CA021661/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA058348/CA/NCI NIH HHS/United States
- U10 CA015488/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

