Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial
- PMID: 22585697
- PMCID: PMC4874149
- DOI: 10.1200/JCO.2011.37.9743
Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial
Abstract
Purpose: To compare the receipt of clofarabine plus cytarabine (Clo+Ara-C arm) with cytarabine (Ara-C arm) in patients ≥ 55 years old with refractory or relapsed acute myelogenous leukemia (AML).
Patients and methods: Patients were randomly assigned to receive either clofarabine (Clo) 40 mg/m(2) or a placebo followed by Ara-C 1 g/m(2) for five consecutive days. The primary end point was overall survival (OS). Secondary end points included event-free survival (EFS), 4-month EFS, overall remission rate (ORR; complete remission [CR] plus CR with incomplete peripheral blood count recovery), disease-free survival (DFS), duration of remission (DOR), and safety.
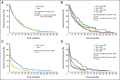
Results: Among 320 patients with confirmed AML (median age, 67 years), the median OS was 6.6 months in the Clo+Ara-C arm and 6.3 months in the Ara-C arm (hazard ratio [HR], 1.00; 95% CI, 0.78 to 1.28; P = 1.00). The ORR was 46.9% in the Clo+Ara-C arm (35.2% CR) versus 22.9% in the Ara-C arm (17.8% CR; P < .01). EFS (HR: 0.63; 95% CI, 0.49 to 0.80; P < .01) and 4-month EFS (37.7% v 16.6%; P < .01) favored the Clo+Ara-C arm compared with Ara-C arm, respectively. DFS and DOR were similar in both arms. Overall 30-day mortality was 16% and 5% for CLO+Ara-C and Ara-C arms, respectively. In the Clo+Ara-C and Ara-C arms, the most common grade 3 to 4 toxicities were febrile neutropenia (47% v 35%, respectively), hypokalemia (18% v 11%, respectively), thrombocytopenia (16% v 17%, respectively), pneumonia (14% v 10%, respectively), anemia (13% v 0%, respectively), neutropenia (11% v 9%, respectively), increased AST (11% v 2%, respectively), and increased ALT (10% v 3%, respectively).
Conclusion: Although the primary end point of OS did not differ between arms, Clo+Ara-C significantly improved response rates and EFS. Study follow-up continues, and the role of clofarabine in the treatment of adult patients with AML continues to be investigated.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Surveillance Epidemiology and End Results. Fast Stats. Acute myeloid leukemia 5-year relative survival by year diagnosis and age at diagnosis/death 1975-2002. 2011. http://www.seer.cancer.gov/faststats/selections.php?series=age.
-
- Altekruse SF, et al. SEER Cancer Statistics Review 1975-2007. National Cancer Institute. 2011. http://seer.cancer.gov/csr/1975_2007/
-
- Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–4116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

