Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung
- PMID: 22585735
- PMCID: PMC3371730
- DOI: 10.1084/jem.20112667
Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung
Abstract
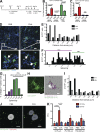
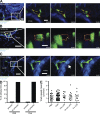
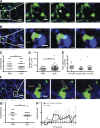
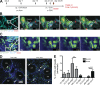
Asthma pathogenesis is focused around conducting airways. The reasons for this focus have been unclear because it has not been possible to track the sites and timing of antigen uptake or subsequent antigen presentation to effector T cells. In this study, we use two-photon microscopy of the lung parenchyma and note accumulation of CD11b(+) dendritic cells (DCs) around the airway after allergen challenge but very limited access of these airway-adjacent DCs to the contents of the airspace. In contrast, we observed prevalent transepithelial uptake of particulate antigens by alveolar DCs. These distinct sites are temporally linked, as early antigen uptake in alveoli gives rise to DC and antigen retention in the airway-adjacent region. Antigen-specific T cells also accumulate in the airway-adjacent region after allergen challenge and are activated by the accumulated DCs. Thus, we propose that later airway hyperreactivity results from selective retention of allergen-presenting DCs and antigen-specific T cells in airway-adjacent interaction zones, not from variation in the abilities of individual DCs to survey the lung.
Figures

Comment in
-
Antigen presentation: Visualizing DC dynamics in the lung.Nat Rev Immunol. 2012 Jun 1;12(7):473. doi: 10.1038/nri3246. Nat Rev Immunol. 2012. PMID: 22653228 No abstract available.
References
-
- Belz G.T., Smith C.M., Kleinert L., Reading P., Brooks A., Shortman K., Carbone F.R., Heath W.R. 2004. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA. 101:8670–8675 10.1073/pnas.0402644101 - DOI - PMC - PubMed
-
- Bergner A., Sanderson M.J. 2003. Airway contractility and smooth muscle Ca(2+) signaling in lung slices from different mouse strains. J. Appl. Physiol. 95:1325–1332, discussion :1314 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

