Uterine development and fertility are dependent on gene dosage of the nuclear receptor coregulator REA
- PMID: 22585830
- PMCID: PMC3404350
- DOI: 10.1210/en.2012-1044
Uterine development and fertility are dependent on gene dosage of the nuclear receptor coregulator REA
Abstract
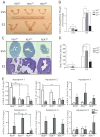
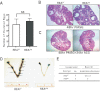
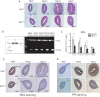
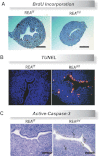
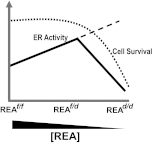
Although the effectiveness of nuclear hormone-receptor complexes is known to depend on coregulator partner proteins, relatively little is known about the roles of coregulators in uterine development and early stages of pregnancy and implantation. Because conventional genetic deletion of the coregulator, repressor of estrogen receptor activity (REA), was embryonic lethal, we here study REA conditional knockout mice generated by cre-loxP recombination, in which REA function was abrogated only in progesterone receptor-expressing tissues, to define the roles of REA in postembryonic stages and in a tissue-specific manner. We find that REA has gene dose-dependent activity impacting uterine development and fertility. Conditional homozygous mutant (REA(d/d)) mice developed to adulthood and showed normal ovarian function, but females were infertile with severely compromised uterine development and function characterized by cell cycle arrest, apoptosis, and altered adenogenesis (endometrial gland morphogenesis), resulting in failure of implantation and decidualization. By contrast, mice heterozygous for REA (REA(f/d)) had a very different phenotype, with estradiol treatment resulting in hyperstimulated, large uteri showing increased proliferation of luminal epithelial cells, and enhanced fluid imbibition associated with altered regulation of aquaporins. These REA(f/d) female mice showed a subfertility phenotype with reduced numbers and sizes of litters. These findings highlight that uterine development and regulation of estrogen receptor activities show a bimodal dependence on the gene dosage of REA. Optimal uterine development and functional activities require the normal gene dosage of REA, with partial or complete deletion resulting in hyperresponsiveness or underresponsiveness to hormone and subfertility or infertility, respectively.
Figures


Similar articles
-
The p160/steroid receptor coactivator family: potent arbiters of uterine physiology and dysfunction.Biol Reprod. 2014 Nov;91(5):122. doi: 10.1095/biolreprod.114.125021. Epub 2014 Oct 8. Biol Reprod. 2014. PMID: 25297546 Free PMC article. Review.
-
The coregulator, repressor of estrogen receptor activity (REA), is a crucial regulator of the timing and magnitude of uterine decidualization.Endocrinology. 2013 Mar;154(3):1349-60. doi: 10.1210/en.2012-2026. Epub 2013 Feb 7. Endocrinology. 2013. PMID: 23392257 Free PMC article.
-
Repressor of estrogen receptor activity (REA) is essential for mammary gland morphogenesis and functional activities: studies in conditional knockout mice.Endocrinology. 2011 Nov;152(11):4336-49. doi: 10.1210/en.2011-1100. Epub 2011 Aug 23. Endocrinology. 2011. PMID: 21862609 Free PMC article.
-
Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo.Mol Cell Biol. 2005 Mar;25(5):1989-99. doi: 10.1128/MCB.25.5.1989-1999.2005. Mol Cell Biol. 2005. PMID: 15713652 Free PMC article.
-
Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human.Nucl Recept Signal. 2007 Nov 30;5:e011. doi: 10.1621/nrs.05011. Nucl Recept Signal. 2007. PMID: 18174919 Free PMC article. Review.
Cited by
-
A murine uterine transcriptome, responsive to steroid receptor coactivator-2, reveals transcription factor 23 as essential for decidualization of human endometrial stromal cells.Biol Reprod. 2014 Apr 10;90(4):75. doi: 10.1095/biolreprod.114.117531. Print 2014 Apr. Biol Reprod. 2014. PMID: 24571987 Free PMC article.
-
Modulation of tumorigenesis by dietary intervention is not mediated by SIRT1 catalytic activity.PLoS One. 2014 Nov 7;9(11):e112406. doi: 10.1371/journal.pone.0112406. eCollection 2014. PLoS One. 2014. PMID: 25380034 Free PMC article.
-
The p160/steroid receptor coactivator family: potent arbiters of uterine physiology and dysfunction.Biol Reprod. 2014 Nov;91(5):122. doi: 10.1095/biolreprod.114.125021. Epub 2014 Oct 8. Biol Reprod. 2014. PMID: 25297546 Free PMC article. Review.
-
Lineage tracing: technology tool for exploring the development, regeneration, and disease of the digestive system.Stem Cell Res Ther. 2020 Oct 15;11(1):438. doi: 10.1186/s13287-020-01941-y. Stem Cell Res Ther. 2020. PMID: 33059752 Free PMC article. Review.
-
Tissue-based quantitative proteomics to screen and identify the potential biomarkers for early recurrence/metastasis of esophageal squamous cell carcinoma.Cancer Med. 2018 Jun;7(6):2504-2517. doi: 10.1002/cam4.1463. Epub 2018 Apr 23. Cancer Med. 2018. PMID: 29683265 Free PMC article.
References
-
- Hewitt SC, Korach KS. 2003. Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction 125:143–149 - PubMed
-
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. 2004. Molecular cues to implantation. Endocr Rev 25:341–373 - PubMed
-
- McKenna NJ, O'Malley BW. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 - PubMed
-
- Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. 2000. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193; discussion 194–165 - PubMed
-
- Delage-Mourroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS. 2000. Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J Biol Chem 275:35848–35856 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

