The transcription factor network associated with the amino acid response in mammalian cells
- PMID: 22585903
- PMCID: PMC3649461
- DOI: 10.3945/an.112.001891
The transcription factor network associated with the amino acid response in mammalian cells
Abstract
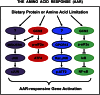
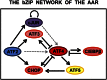
Mammals exhibit multiple adaptive mechanisms that sense and respond to fluctuations in dietary nutrients. Consumption of reduced total dietary protein or a protein diet that is deficient in 1 or more of the essential amino acids triggers wide-ranging changes in feeding behavior and gene expression. At the level of individual cells, dietary protein deficiency is manifested as amino acid (AA) deprivation, which activates the AA response (AAR). The AAR is composed of a collection of signal transduction pathways that terminate in specific transcriptional programs designed to catalyze adaptation to the nutrient stress or, ultimately, undergo apoptosis. Independently of the AAR, endoplasmic reticulum stress activates 3 signaling pathways, collectively referred to as the unfolded protein response. The transcription factor activating transcription factor 4 is one of the terminal transcriptional mediators for both the AAR and the unfolded protein response, leading to a significant degree of overlap with regard to the target genes for these stress pathways. Over the past 5 y, research has revealed that the basic leucine zipper superfamily of transcription factors plays the central role in the AAR. Formation of both homo- and heterodimers among the activating transcription factor, CCAAT enhancer-binding protein, and FOS/JUN families of basic leucine zipper proteins forms the nucleus of a highly integrated transcription factor network that determines the initiation, magnitude, and duration of the cellular response to dietary protein or AA limitation.
Conflict of interest statement
Author disclosures: M. S. Kilberg, M. Balasubramanian, L. Fu, and J. Shan, no conflicts of interest.
Figures
Similar articles
-
ATF4-dependent transcription mediates signaling of amino acid limitation.Trends Endocrinol Metab. 2009 Nov;20(9):436-43. doi: 10.1016/j.tem.2009.05.008. Epub 2009 Sep 30. Trends Endocrinol Metab. 2009. PMID: 19800252 Free PMC article. Review.
-
Auto-activation of c-JUN gene by amino acid deprivation of hepatocellular carcinoma cells reveals a novel c-JUN-mediated signaling pathway.J Biol Chem. 2011 Oct 21;286(42):36724-38. doi: 10.1074/jbc.M111.277673. Epub 2011 Aug 23. J Biol Chem. 2011. PMID: 21862593 Free PMC article.
-
Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) System A transporter gene.Biochem J. 2006 May 1;395(3):517-27. doi: 10.1042/BJ20051867. Biochem J. 2006. PMID: 16445384 Free PMC article.
-
Expression profiling after activation of amino acid deprivation response in HepG2 human hepatoma cells.Physiol Genomics. 2010 May;41(3):315-27. doi: 10.1152/physiolgenomics.00217.2009. Epub 2010 Mar 9. Physiol Genomics. 2010. PMID: 20215415 Free PMC article.
-
Amino acid limitation regulates gene expression.Proc Nutr Soc. 1999 Aug;58(3):625-32. doi: 10.1017/s0029665199000828. Proc Nutr Soc. 1999. PMID: 10604196 Review.
Cited by
-
CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis.Mol Biol Cell. 2013 Aug;24(15):2477-90. doi: 10.1091/mbc.E13-01-0067. Epub 2013 Jun 12. Mol Biol Cell. 2013. PMID: 23761072 Free PMC article.
-
Regulatory crosstalk within the mammalian unfolded protein response.Cell Mol Life Sci. 2014 Mar;71(6):1067-79. doi: 10.1007/s00018-013-1490-2. Epub 2013 Oct 18. Cell Mol Life Sci. 2014. PMID: 24135849 Free PMC article. Review.
-
General amino acid control in fission yeast is regulated by a nonconserved transcription factor, with functions analogous to Gcn4/Atf4.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):E1829-E1838. doi: 10.1073/pnas.1713991115. Epub 2018 Feb 5. Proc Natl Acad Sci U S A. 2018. PMID: 29432178 Free PMC article.
-
Asparagine Synthetase Deficiency causes reduced proliferation of cells under conditions of limited asparagine.Mol Genet Metab. 2015 Nov;116(3):178-86. doi: 10.1016/j.ymgme.2015.08.007. Epub 2015 Aug 14. Mol Genet Metab. 2015. PMID: 26318253 Free PMC article.
-
Quantitative modelling of amino acid transport and homeostasis in mammalian cells.Nat Commun. 2021 Sep 6;12(1):5282. doi: 10.1038/s41467-021-25563-x. Nat Commun. 2021. PMID: 34489418 Free PMC article.
References
-
- Gietzen DW, Rogers QR. Nutritional homeostasis and indispensable amino acid sensing: a new solution to an old puzzle. Trends Neurosci. 2006;29:91–9 - PubMed
-
- Koehnle TJ, Russell MC, Morin AS, Erecius LF, Gietzen DW. Diets deficient in indispensable amino acids rapidly decrease the concentration of the limiting amino acid in the anterior piriform cortex of rats. J Nutr. 2004;134:2365–71 - PubMed
-
- Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–8 - PubMed
-
- Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–7 - PubMed
-
- Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–14 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

