Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons
- PMID: 22586100
- PMCID: PMC3384192
- DOI: 10.1073/pnas.1118715109
Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons
Abstract
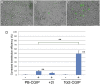
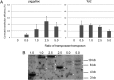
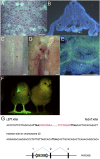
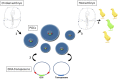
The derivation of germ-line competent avian primordial germ cells establishes a cell-based model system for the investigation of germ cell differentiation and the production of genetically modified animals. Current methods to modify primordial germ cells using DNA or retroviral vectors are inefficient and prone to epigenetic silencing. Here, we validate the use of transposable elements for the genetic manipulation of primordial germ cells. We demonstrate that chicken primordial germ cells can be modified in vitro using transposable elements. Both piggyBac and Tol2 transposons efficiently transpose primordial germ cells. Tol2 transposon integration sites were spread throughout both the macro- and microchromosomes of the chicken genome and were more prevalent in gene transcriptional units and intronic regions, consistent with transposon integrations observed in other species. We determined that the presence of insulator elements was not required for reporter gene expression from the integrated transposon. We further demonstrate that a gene-trap cassette carried in the Tol2 transposon can trap and mutate endogenous transcripts in primordial germ cells. Finally, we observed that modified primordial germ cells form functional gametes as demonstrated by the generation of transgenic offspring that correctly expressed a reporter gene carried in the transposon. Transposable elements are therefore efficient vectors for the genetic manipulation of primordial germ cells and the chicken genome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Karagenç L, Cinnamon Y, Ginsburg M, Petitte JN. Origin of primordial germ cells in the prestreak chick embryo. Dev Genet. 1996;19:290–301. - PubMed
-
- Kagami H, et al. The developmental origin of primordial germ cells and the transmission of the donor-derived gametes in mixed-sex germline chimeras to the offspring in the chicken. Mol Reprod Dev. 1997;48:501–510. - PubMed
-
- Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127:2741–2750. - PubMed
-
- Nieuwkoop PD, Sutasurya LA. Primordal Germ Cells in the Chordates. Cambridge, UK: Cambridge Univ Press; 1979.
-
- van de Lavoir MC, et al. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

