The BATTLE trial: personalizing therapy for lung cancer
- PMID: 22586319
- PMCID: PMC4211116
- DOI: 10.1158/2159-8274.CD-10-0010
The BATTLE trial: personalizing therapy for lung cancer
Abstract
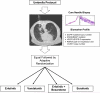
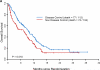
The Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial represents the first completed prospective, biopsy-mandated, biomarker-based, adaptively randomized study in 255 pretreated lung cancer patients. Following an initial equal randomization period, chemorefractory non-small cell lung cancer (NSCLC) patients were adaptively randomized to erlotinib, vandetanib, erlotinib plus bexarotene, or sorafenib, based on relevant molecular biomarkers analyzed in fresh core needle biopsy specimens. Overall results include a 46% 8-week disease control rate (primary end point), confirm prespecified hypotheses, and show an impressive benefit from sorafenib among mutant-KRAS patients. BATTLE establishes the feasibility of a new paradigm for a personalized approach to lung cancer clinical trials.
Significance: The BATTLE study is the first completed prospective, adaptively randomized study in heavily pretreated NSCLC patients that mandated tumor profiling with "real-time" biopsies, taking a substantial step toward realizing personalized lung cancer therapy by integrating real-time molecular laboratory findings in delineating specific patient populations for individualized treatment.
Trial registration: ClinicalTrials.gov NCT00409968 NCT00410059 NCT00410189 NCT00411632 NCT00411671.
Figures


Comment in
-
A new BATTLE in the evolving war on cancer.Cancer Discov. 2011 Jun;1(1):14-6. doi: 10.1158/2159-8274.CD-11-0044. Epub 2011 Jun 1. Cancer Discov. 2011. PMID: 22586313
-
The BATTLE trial: a bold step toward improving the efficiency of biomarker-based drug development.Cancer Discov. 2011 Jun;1(1):17-20. doi: 10.1158/2159-8274.CD-11-0036. Epub 2011 Jun 1. Cancer Discov. 2011. PMID: 22586314
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Jul 7; [Epub ahead of print] - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. PMID: 17167137. - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. PMID: 18506025. - PubMed
-
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small cell lung cancer (INTEREST): a randomized Phase III trial. Lancet. 2008;372:1809–1818. PMID: 19027483. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

