Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial
- PMID: 22586334
- PMCID: PMC3394820
- DOI: 10.1503/cmaj.110837
Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial
Abstract
Background: Spasticity is a common and poorly controlled symptom of multiple sclerosis. Our objective was to determine the short-term effect of smoked cannabis on this symptom.
Methods: We conducted a placebo-controlled, crossover trial involving adult patients with multiple sclerosis and spasticity. We recruited participants from a regional clinic or by referral from specialists. We randomly assigned participants to either the intervention (smoked cannabis, once daily for three days) or control (identical placebo cigarettes, once daily for three days). Each participant was assessed daily before and after treatment. After a washout interval of 11 days, participants crossed over to the opposite group. Our primary outcome was change in spasticity as measured by patient score on the modified Ashworth scale. Our secondary outcomes included patients' perception of pain (as measured using a visual analogue scale), a timed walk and changes in cognitive function (as measured by patient performance on the Paced Auditory Serial Addition Test), in addition to ratings of fatigue.
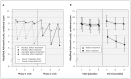
Results: Thirty-seven participants were randomized at the start of the study, 30 of whom completed the trial. Treatment with smoked cannabis resulted in a reduction in patient scores on the modified Ashworth scale by an average of 2.74 points more than placebo (p < 0.0001). In addition, treatment reduced pain scores on a visual analogue scale by an average of 5.28 points more than placebo (p = 0.008). Scores for the timed walk did not differ significantly between treatment and placebo (p = 0.2). Scores on the Paced Auditory Serial Addition Test decreased by 8.67 points more with treatment than with placebo (p = 0.003). No serious adverse events occurred during the trial.
Interpretation: Smoked cannabis was superior to placebo in symptom and pain reduction in participants with treatment-resistant spasticity. Future studies should examine whether different doses can result in similar beneficial effects with less cognitive impact.
Figures

References
-
- Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry 2000;57:547–52 - PubMed
-
- Brooks JW, Pryce G, Bisogno T, et al. Arvanil-induced inhibition of spasticity and persistent pain: evidence for therapeutic sites of action different from the vanilloid VR1 receptor and cannabinoid CB(1)/CB(2) receptors. Eur J Pharmacol 2002; 439:83–92 - PubMed
-
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–7 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
