The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide
- PMID: 22586420
- PMCID: PMC3343455
- DOI: 10.3389/fmicb.2012.00154
The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide
Abstract
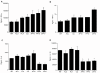
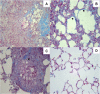
Paracoccidioidomycosis (PCM), a common chronic mycosis in Latin America, is a granulomatous systemic disease caused by the thermo-dimorphic fungus Paracoccidioides brasiliensis. The glycoprotein gp43 is the main antigen target of P. brasiliensis and a 15-mer internal peptide (QTLIAIHTLAIRYAN), known as P10, defines a major CD4(+)-specific T cell epitope. Previous results have indicated that, besides having a preventive role in conventional immunizations prior to challenge with the fungus, protective anti-fungal effects can be induced in P. brasiliensis-infected mice treated with P10 administered with complete Freund's adjuvant (CFA). The peptide elicits an IFN-γ-dependent Th1 immune response and is the main candidate for effective immunotherapy of patients with PCM, as an adjunctive approach to conventional chemotherapy. In the present study we tested the therapeutic effects of P10 combined with different adjuvants [aluminum hydroxide, CFA, flagellin, and the cationic lipid dioctadecyl-dimethylammonium bromide (DODAB)] in BALB/c mice previously infected with the P. brasiliensis Pb18 strain. Significant reductions in the number of colony forming units of the fungus were detected in lungs of mice immunized with P10 associated with the different adjuvants 52 days after infection. Mice treated with DODAB and P10, followed by mice treated with P10 and flagellin, showed the most prominent effects as demonstrated by the lowest numbers of viable yeast cells as well as reductions in granuloma formation and fibrosis. Concomitantly, secretion of IFN-γ and TNF-α, in contrast to interleukin (IL)-4 and IL-10, was enhanced in the lungs of mice immunized with P10 in combination with the tested adjuvants, with the best results observed in mice treated with P10 and DODAB. In conclusion, the present results demonstrate that the co-administration of the synthetic P10 peptide with several adjuvants, particularly DODAB, have significant therapeutic effects in experimental PCM.
Keywords: FliC flagellin; P10; Paracoccidioides brasiliensis; adjuvants; aluminum hydroxide; complete Freund’s adjuvant; dioctadecyl-dimethylammonium bromide; paracoccidioidomycosis.
Figures
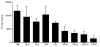

References
-
- Amaral A. C., Marques A. F., Muñoz J. E., Bocca A. L., Simioni A. R., Tedesco A. C., Morais P. C., Travassos L. R., Taborda C. P., Felipe M. S. (2010). Poly(lactic acid-glycolic acid) nanoparticles markedly improve immunological protection provided by peptide P10 against murine paracoccidioidomycosis. Br. J. Pharmacol. 159 1126–1132 - PMC - PubMed
-
- Baylor N. M., Egan W., Richman P. (2002). Aluminum salts in vaccines – US perspective. Vaccine 20 18–23 - PubMed
-
- Blotta M. H., Mamoni R. L., Oliveira S. J., Nouer S. A., Papaiordanou P. M., Goveia A., Camargo Z. P. (1999). Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am. J. Trop. Med. Hyg. 61 390–394 - PubMed
-
- Buissa-Filho R., Puccia R., Marques A. F., Pinto F. A., Muñoz J. E., Nosanchuk J. D., Travassos L. R., Taborda C. P. (2008). The monoclonal antibody against the major diagnostic antigen of Paracoccidioides brasiliensis mediates immune protection in infected BALB/c mice challenged intratracheally with the fungus. Infect. Immun. 76 3321–3328 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

