Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem
- PMID: 22586427
- PMCID: PMC3347682
- DOI: 10.3389/fimmu.2012.00087
Pandemic H1N1 Influenza Infection and Vaccination in Humans Induces Cross-Protective Antibodies that Target the Hemagglutinin Stem
Abstract
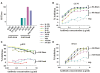
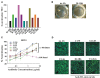
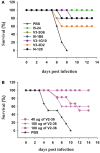
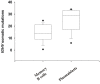
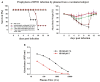
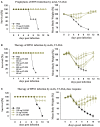
Most monoclonal antibodies (mAbs) generated from humans infected or vaccinated with the 2009 pandemic H1N1 (pdmH1N1) influenza virus targeted the hemagglutinin (HA) stem. These anti-HA stem mAbs mostly used IGHV1-69 and bound readily to epitopes on the conventional seasonal influenza and pdmH1N1 vaccines. The anti-HA stem mAbs neutralized pdmH1N1, seasonal influenza H1N1 and avian H5N1 influenza viruses by inhibiting HA-mediated fusion of membranes and protected against and treated heterologous lethal infections in mice with H5N1 influenza virus. This demonstrated that therapeutic mAbs could be generated a few months after the new virus emerged. Human immunization with the pdmH1N1 vaccine induced circulating antibodies that when passively transferred, protected mice from lethal, heterologous H5N1 influenza infections. We observed that the dominant heterosubtypic antibody response against the HA stem correlated with the relative absence of memory B cells against the HA head of pdmH1N1, thus enabling the rare heterosubtypic memory B cells induced by seasonal influenza and specific for conserved sites on the HA stem to compete for T-cell help. These results support the notion that broadly protective antibodies against influenza would be induced by successive vaccination with conventional influenza vaccines based on subtypes of HA in viruses not circulating in humans.
Keywords: competition for T-cell help; cross-protective antibodies; hemagglutinin; heterosubtypic; memory B cells; pandemic H1N1 influenza; plasmablasts; vaccines.
Figures

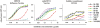
References
-
- Babcook J. S., Leslie K. B., Olsen O. A., Salmon R. A., Schrader J. W. (1996). A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc. Natl. Acad. Sci. U.S.A. 93, 7843–784810.1073/pnas.93.15.7843 - DOI - PMC - PubMed
-
- Barington T., Heilmann C., Andersen V. (1990). Quantitation of antibody-secreting cells in the blood after vaccination with Haemophilus influenzae type b conjugate vaccine. Scand. J. Immunol. 31, 515–522 [Published erratum appears in Scand. J. Immunol 32, 59].10.1111/j.1365-3083.1990.tb02799.x - DOI - PubMed
-
- Corti D., Suguitan A. L., Pinna D., Silacci C., Fernandez-Rodriguez B. M., Vanzetta F., Santos C., Luke C. J., Torres-Velez F. J., Temperton N. J., Weiss R. A., Sallusto F., Subbarao K., Lanzavecchia A. (2010). Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120, 1663–167310.1172/JCI41902 - DOI - PMC - PubMed
-
- Corti D., Voss J., Gamblin S. J., Codoni G., Macagno A., Jarrossay D., Vachieri S. G., Pinna D., Minola A., Vanzetta F., Silacci C., Fernandez-Rodriguez B. M., Agatic G., Bianchi S., Giacchetto-Sasselli I., Calder L., Sallusto F., Collins P., Haire L. F., Temperton N., Langedijk J. P. M., Skehel J. J., Lanzavecchia A. (2011). A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza a hemagglutinins. Science 333, 850–85610.1126/science.1205669 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

