The prevalence of immunologic injury in renal allograft recipients with de novo proteinuria
- PMID: 22586485
- PMCID: PMC3346732
- DOI: 10.1371/journal.pone.0036654
The prevalence of immunologic injury in renal allograft recipients with de novo proteinuria
Abstract
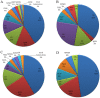
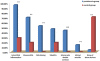
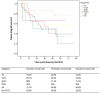
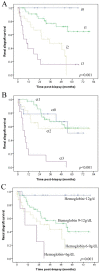
Post-transplant proteinuria is a common complication after renal transplantation; it is associated with reduced graft and recipient survival. However, the prevalence of histological causes has been reported with considerable variation. A clinico-pathological re-evaluation of post-transplant proteinuria is necessary, especially after dismissal of the term "chronic allograft nephropathy," which had been considered to be an important cause of proteinuria. Moreover, urinary protein can promote interstitial inflammation in native kidney, whether this occurs in renal allograft remains unknown. Factors that affect the graft outcome in patients with proteinuria also remain unclear. Here we collected 98 cases of renal allograft recipients who developed proteinuria after transplant, histological features were characterized using Banff scoring system. Cox proportional hazard regression models were used for graft survival predictors. We found that transplant glomerulopathy was the leading (40.8%) cause of post-transplant proteinuria. Immunological causes, including transplant glomerulopathy, acute rejection, and chronic rejection accounted for the majority of all pathological causes of proteinuria. Nevertheless, almost all patients that developed proteinuria had immunological lesions in the graft, especially for interstitial inflammation. Intraglomerular C3 deposition was unexpectedly correlated with the severity of proteinuria. Moreover, the severity of interstitial inflammation was an independent risk factor for graft loss, while high level of hemoglobin was a protective factor for graft survival. This study revealed a predominance of immunological parameters in renal allografts with post-transplant proteinuria. These parameters not only correlate with the severity of proteinuria, but also with the outcome of the graft.
Conflict of interest statement
Figures
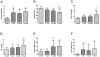
References
-
- Hohage H, Kleyer U, Bruckner D, August C, Zidek W, et al. Influence of proteinuria on long-term transplant survival in kidney transplant recipients. Nephron. 1997;75:160–165. - PubMed
-
- Amer H, Fidler ME, Myslak M, Morales P, Kremers WK, et al. Proteinuria after kidney transplantation, relationship to allograft histology and survival. Am J Transplant. 2007;7:2748–2756. - PubMed
-
- Yakupoglu U, Baranowska-Daca E, Rosen D, Barrios R, Suki WN, et al. Post-transplant nephrotic syndrome: A comprehensive clinicopathologic study. Kidney Int. 2004;65:2360–2370. - PubMed
-
- Halimi J-M, Laouad I, Buchler M, Al-Najjar A, Chatelet V, et al. Early Low-Grade Proteinuria: Causes, Short-Term Evolution and Long-Term Consequences in Renal Transplantation. American Journal of Transplantation. 2005;5:2281–2288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

